- 1 Unlock Trusted MDAI Purchasing Options – Safe & Accessible
- 2 Explore MDAI for Sale with Confidence
- 3 Factors to Consider When Buying MDAI
- 4 Why Choose MDAI?
- 5 Secure Your MDAI Today
- 6 Summary
- 7 Chemistry
- 8 Pharmacology
- 9 Subjective effects
- 10 Toxicity
- 11 Legal status
- 12 FAQ
- 12.1 1. What is MDAI?
- 12.2 2. What are entactogenic effects?
- 12.3 3. Is MDAI legal?
- 12.4 4. How is MDAI typically consumed?
- 12.5 5. What are the effects of MDAI?
- 12.6 6. Is MDAI safe to use?
- 12.7 7. Can MDAI be addictive?
- 12.8 8. Are there any dangerous interactions with MDAI?
- 12.9 9. What is the recommended dosage of MDAI?
- 12.10 10. Are there any long-term effects associated with MDAI use?
- 13 References
Unlock Trusted MDAI Purchasing Options – Safe & Accessible
When looking to buy MDAI, finding trustworthy vendors and ensuring reliable options are vital. Whether you’re browsing buy MDAI online platforms from the USA, Canada, or elsewhere, understanding your choices can enhance your experience. With options like MDAI for sale from global suppliers and specialized MDAI shops, there has never been a more accessible time to find this popular product.
Explore MDAI for Sale with Confidence
MDAI has proven itself as a preferred choice in the research chemical sector. Packed with engaging features, buyers appreciate the availability of MDAI online in various forms and packaging options. Trusted platforms provide simple solutions to purchase MDAI globally, whether you’re in search of buy MDAI USA opportunities, MDAI Canada suppliers, or international markets catering to MDAI vendors.
Users seeking an easy way to buy MDAI online can benefit from vendors offering secure payment systems, such as credit card acceptance. Many reputable sellers prioritize product descriptions and purity transparency—ensuring that what you order matches the exact specifications you need.
Factors to Consider When Buying MDAI
Before finalizing your MDAI buy decision, consider these essential tips to ensure safety and quality:
- Reliable Vendors
Look for vendors with verified reviews and a strong track record in the research chemical industry. Reputable suppliers often feature detailed labels and test reports.
- Global Shipping
Whether you’re in the USA, Canada, or even looking to buy MDAI Australia, select sellers who provide discreet and secure shipping methods to your location.
- Pricing and Payment Options
Compare pricing across multiple MDAI sale offers to find fair rates. Trusted sources also accept a range of payment methods for your convenience.
Why Choose MDAI?
Many choose MDAI research chemicals for its broad applications and consistent quality offered by leading suppliers. Its growing popularity is matched by the convenience of buying it online, allowing you to browse options from trusted MDAI shop platforms. Whether you need products for research or specialized requirements, the variety of sellers ensures tailored results.
For frequent buyers, being able to order MDAI online quickly and securely adds unmatched convenience. From small-scale purchases to bulk supplies, major platforms provide significant flexibility.
Secure Your MDAI Today
With a rise in global platforms offering MDAI for sale, purchasing this compound has never been easier—or safer. Whether you’re exploring options to buy MDAI Canada, the USA, or internationally, prioritize platforms that promise authenticity, safety, and a seamless shopping experience. Take advantage of today’s verified suppliers and ensure your next MDAI purchase is secure and reliable.
Summary
5,6-Methylenedioxy-2-aminoindane, commonly referred to as MDAI, is a relatively lesser-known entactogen belonging to the aminoindane class. It is recognized for its unique properties as a selective serotonin-releasing agent (SSRA) with minimal impact on dopamine and norepinephrine neurotransmitters.
MDAI was initially developed by the American medicinal chemist and pharmacologist David E. Nichols in the 1990s while at Purdue University. In the 2010s, MDAI gained attention and found its place in the online research chemical market, often alongside other novel entactogens like 5-MAPB, 5-APB, and 6-APB. During this time, it was marketed as a legal, grey-market alternative to MDMA. Moreover, researchers are exploring its potential application as an alternative to conventional entactogens in treating mental health conditions such as anxiety and depression.
Users of MDAI report a range of subjective effects, including sedation, muscle relaxation, enhanced tactile perception, anxiety alleviation, increased empathy, sociability, and euphoria. It is often likened to producing the non-stimulating effects characteristic of prototypical entactogens like MDMA and MDA. Notably, MDAI is believed to have reduced invigorating, highly prosocial, or intensely euphoric results, potentially offering the advantage of reduced neurotoxicity and fewer side effects. Nevertheless, further research is essential to substantiate these claims.
Given the limited available data regarding MDAI’s pharmacological properties, metabolism, and toxicity, it is strongly recommended that individuals considering its use harm reduction practices be employed.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 132741-81-2 |
|---|---|
| PubChem CID | 125558 |
| ChemSpider | 111694 |
| UNII | 0DMJ6G3XBF |
| CompTox Dashboard (EPA) | DTXSID60157741 |
| Chemical and physical data | |
| Formula | C10H11NO2 |
| Molar mass | 177.203 g·mol− |
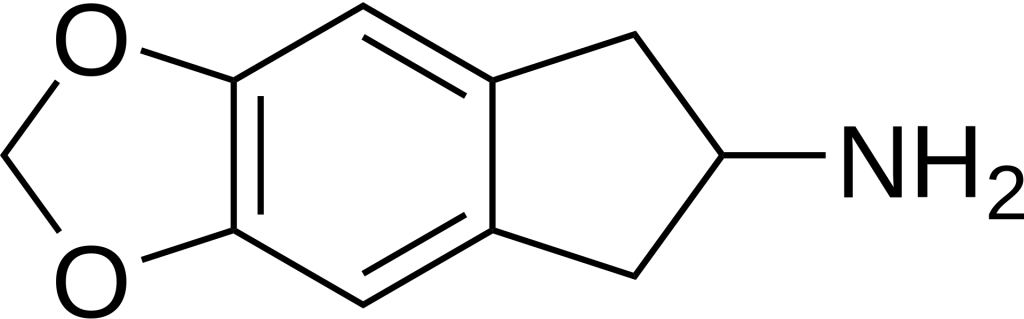
Chemistry
MDAI, scientifically known as 5,6-methylenedioxy-2-aminoindane, is a synthetic compound classified within the aminoindane family and shares structural similarities with amphetamines. In its molecular structure, MDAI possesses the R3 terminal carbon of the amphetamine’s propane chain, which is bonded to a benzene ring. This unique arrangement forms an indane group characterized by the fusion of a benzene ring with a pentane ring. Notably, MDAI incorporates an amino group (NH2) attached to R2 of the indane ring. Additionally, the compound features two oxygen substitutions at R5 and R6, connected via a methylene bridge, thus forming a methylenedioxy group.
MDAI can be likened to its structural relative, 2-AI, with the key distinction being the presence of a methylenedioxy ring within MDAI’s molecular structure.
Pharmacology
MDAI can impede serotonin reuptake and demonstrates a particular affinity for serotonin receptors. Scientific investigations have revealed that animals treated with MDAI experience elevated extracellular levels of monoamine neurotransmitters, notably serotonin, within their brains.
In a comparative context, MDAI’s potency in serotonin release is akin to that of MDA but notably less potent than MDMA. This mechanism involves the inhibition of neurotransmitter reuptake and reabsorption after they have fulfilled their role in transmitting neural impulses. Consequently, this inhibition allows these neurotransmitters to accumulate, be recycled, and induce entactogenic effects.
Subjective effects
Disclaimer: The following effects are based on anecdotal user reports and the insights of PsychonautWiki contributors, as documented in the Subjective Effect Index (SEI). They should be approached with a degree of scepticism. It’s important to note that these effects may only sometimes manifest predictably. Higher doses are more likely to induce a broader range of products, and escalating doses increase the risk of adverse effects, including addiction, severe harm, or even fatality ☠.
Physical :
- Sedation – A distinguishing feature of MDAI is its tendency to induce moderate sedation, discouraging physically active behaviours like running, dancing, or climbing.
- Spontaneous physical sensations – MDAI produces a moderate to extreme euphoric, warm, and tingling sensation that envelops the entire body. This sensation becomes intensely pleasurable at higher doses.
Physical euphoria:
- Tactile enhancement
- Vibrating vision – At higher doses, eyeballs may exhibit spontaneous rapid movements, causing temporary blurriness and a phenomenon known as nystagmus.
- Increased perspiration
- Temperature regulation suppression
- Dehydration – Dry mouth and dehydration are common effects, driven by an elevated heart rate and a strong urge to engage in vigorous physical activities. To avoid over-drinking, it’s advisable to sip water instead.
- Difficulty urinating – Like MDMA, higher doses of MDAI can lead to temporary and harmless difficulty urinating. This is likely due to the anti-diuretic hormone (ADH) release, which regulates urination. Relaxation, warmth application to the genitals, or the sound of running water may alleviate this.
- Temporary erectile dysfunction
Visual:
- Visual effects are subtle and occur primarily at higher doses, including:
- Visual acuity enhancement
- Colour enhancement
- Tracers
Cognitive:
- The cognitive effects intensify with dosage and are characterized by euphoria, love, and empathy. Essential cognitive products include:
- Anxiety suppression
- Cognitive euphoria – Strong emotional happiness stemming from serotonin release.
- Empathy, affection, and sociability enhancement – Not as pronounced as MDMA but still dominant.
- Increased music appreciation
- Immersion enhancement
- Thought deceleration
- Mindfulness
- Time distortion
Auditory:
- Auditory effects may include enhancements and distortions.
After:
- The post-peak phase, often called the “comedown,” is characterized by adverse and uncomfortable effects due to neurotransmitter depletion. Common after-effects include:
- Anxiety
- Cognitive fatigue
- Depression
- Irritability
- Motivation suppression
- Thought deceleration
- Wakefulness
Toxicity
Disclaimer: The information below is based on anecdotal user reports and the collective insights of PsychonautWiki contributors. While valuable, these insights should be approached with caution. Individual reactions may vary, and higher doses carry increased risks, including addiction and severe harm or fatality ☠.
Pharmacology and Toxicity:
- MDAI and related substances have been widely employed in scientific research because they mimic many of MDMA’s effects without causing neurotoxicity. However, there have been no tests specifically addressing cardiovascular toxicity.[2][3][4][5][6][7][8]
- The lethal dose of MDAI in humans remains unknown, and the exact toxic dosage is yet to be determined. Due to its action as a serotonin reuptake inhibitor, overdosing may lead to serotonin syndrome.
- Harm reduction practices are strongly advised when using this substance.
Dependence and Abuse Potential:
- While formal studies are lacking, MDAI may have a mild addictive potential with a low likelihood of causing psychological dependence in most users. This is due to its unique mechanism of action that does not significantly increase dopamine levels. Those experiencing addiction may encounter cravings and withdrawal symptoms upon cessation.
- Tolerance to MDAI’s effects develops with sustained use, prompting users to administer larger doses for the same results. Afterwards, tolerance reduction takes approximately 3-7 days to reach half and 1-2 weeks to return to baseline (without further consumption).
- MDAI demonstrates cross-tolerance with all entactogens, meaning its use will reduce the effects of other serotonergic stimulants.
Dangerous Interactions:
- Combining psychoactive substances can be perilous, as effects can become life-threatening. Below are some known hazardous interactions. Independent research should always be conducted to ensure safety. Some exchanges are sourced from TripSit:
- 25x-NBOMe & 25x-NBOH – Avoid combining 25x compounds with MDAI due to the risk of excessive stimulation, heart strain, high blood pressure, vasoconstriction, panic attacks, thought loops, seizures, and even heart failure in severe cases.
- Alcohol – Mixing alcohol with stimulants is dangerous because stimulants mask alcohol’s depressant effects. When the motivation wears off, depressant results can take over, leading to blackouts and severe respiratory depression. If combined, strictly limit alcohol intake.
- DXM – Avoid DXM combinations as they inhibit serotonin and norepinephrine reuptake, increasing the risk of panic attacks, hypertensive crisis, or serotonin syndrome with serotonin releasers like MDMA, methylone, or mephedrone. Monitor blood pressure and avoid strenuous activity.
- MDMA – Combining stimulants with MDMA may intensify neurotoxic effects, raise blood pressure, and strain the heart (cardiotoxicity).
- MXE – Some reports suggest that MXE combinations may dangerously elevate blood pressure and risk mania and psychosis.
- Dissociatives – Both classes risk delusions, mania, and psychosis, which may be exacerbated when combined.
- Stimulants – Combining MDAI with other stimulants like cocaine can raise heart rate and blood pressure to dangerous levels.
- Tramadol – Tramadol lowers the seizure threshold, and combining it with stimulants may further increase this risk.
- Cocaine – Combining cocaine with MDAI can excessively strain the heart.
Serotonin Syndrome Risk:
- Combinations with certain substances can elevate serotonin levels to dangerous levels, potentially causing a life-threatening serotonin syndrome. These substances include:
- MAOIs – Such as banisteriopsis caapi, Syrian rue, phenelzine, selegiline, and moclobemide.
- Serotonin Releasers include MDMA, 4-FA, methamphetamine, methylone, and αMT.
- SSRIs – Such as citalopram and sertraline.
- SNRIs – Such as tramadol and venlafaxine.
- 5-HTP
Legal status
Austria: The possession, production, and sale of MDAI are prohibited under the Neue-Psychoaktive-Substanzen-Gesetz Österreich (NPSG).
Brazil: MDAI is illegal for possession, production, and sale, according to Portaria SVS/MS nº 344.
China: As of October 2015, China classifies MDAI as a controlled substance.
Denmark: Since September 2015, MDAI is illegal in Denmark.
Germany: MDAI falls under the regulations of the New Psychoactive Substances Act (NpSG) as of November 26, 2016. Production, import for marketing, administration to others, and trading are punishable offences, while possession is illegal but not penalized.
Japan: As of March 30, 2013, MDAI is considered a controlled substance in Japan.]
Switzerland: MDAI is listed as a controlled substance in Verzeichnis E and has held this status since December 2011.
Turkey: Classified as a drug, MDAI is illegal for possession, production, supply, and import.
United Kingdom: The production, supply, or import of MDAI is prohibited under the Psychoactive Substance Act, effective May 26, 2016.
FAQ
1. What is MDAI?
MDAI, or 5,6-methylenedioxy-2-aminoindane, is a synthetic substance belonging to the aminoindane class. It shares structural similarities with amphetamines and is known for its entactogenic effects.
2. What are entactogenic effects?
Entactogenic effects refer to the ability of a substance to enhance feelings of empathy, emotional closeness, and sociability, often associated with substances like MDMA (Ecstasy).
3. Is MDAI legal?
The legal status of MDAI varies by country. It is illegal in several nations, while others may regulate it differently. Always check your local laws and regulations regarding MDAI before obtaining or using it.
4. How is MDAI typically consumed?
MDAI is usually taken orally as a powder, capsule, or tablet. Users may swallow it directly or mix it with a beverage. Dosage varies, so it’s crucial to measure accurately.
5. What are the effects of MDAI?
MDAI can induce feelings of sedation, muscle relaxation, tactile enhancement, enhanced empathy, sociability, euphoria, and, at higher doses, subtle visual effects. However, individual experiences may vary.
6. Is MDAI safe to use?
The safety of MDAI is not well-established, as limited scientific research has been conducted. Like many psychoactive substances, it carries risks, mainly when misused or in excessive amounts. Harm reduction practices are strongly advised.
7. Can MDAI be addictive?
While MDAI may have a low potential for abuse and psychological dependence compared to traditional stimulants, it can still be habit-forming for some individuals. Cravings and withdrawal effects may occur with regular use.
8. Are there any dangerous interactions with MDAI?
Yes, there are known dangerous interactions with other substances. Combining MDAI with certain drugs, such as stimulants or MAOIs, can lead to severe health risks, including serotonin syndrome. Always research potential interactions before use.
9. What is the recommended dosage of MDAI?
Dosage can vary widely depending on individual tolerance and body weight. It’s essential to start with a low dose, typically between 50-100 milligrams, and wait to assess its effects before considering additional amounts. Stay within recommended dosages.
10. Are there any long-term effects associated with MDAI use?
Due to the limited research on MDAI, long-term effects are not well-documented. It’s essential to prioritize your safety and use this substance responsibly, considering the potential risks associated with any psychoactive compound.
References
- Johnson, M. P., Conarty, P. F., Nichols, D. E. (July 23, 1991). “Neurochemical Properties of 3,4-Methylenedioxymethamphetamine and Analogues.” European Journal of Pharmacology. 200 (1): 9–16. doi:10.1016/0014-2999(91)90659-e. ISSN 0014-2999.
- Nichols, D. E., Brewster, W. K., Johnson, M. P., Oberlender, R., Riggs, R. M. (February 1990). “Tetralin and Indan Analogues of MDA.” Journal of Medicinal Chemistry. 33 (2): 703–710. doi:10.1021/jm00164a037. ISSN 0022-2623.
- Nichols, D. E., Johnson, M. P., Oberlender, R. (January 1991). “Nonneurotoxic Analogue of p-Iodoamphetamine.” Pharmacology, Biochemistry, and Behavior. 38 (1): 135–139. doi:10.1016/0091-3057(91)90601-w. ISSN 0091-3057.
- Johnson, M. P., Conarty, P. F., Nichols, D. E. (May 1, 1991). “Synthesis and Pharmacological Examination of Novel Compounds.” Journal of Medicinal Chemistry. 34 (5): 1662–1668. doi:10.1021/JM00109A020.
- Johnson, M. P., Huang, X. M., Nichols, D. E. (December 1991). “Serotonin Neurotoxicity in Rats After Combined Treatment with Dopaminergic Agents.” Pharmacology, Biochemistry, and Behavior. 40 (4): 915–922. doi:10.1016/0091-3057(91)90106-c. ISSN 0091-3057.
- Nichols, D. E., Marona-Lewicka, D., Huang, X., Johnson, M. P. (1993). “Novel Serotonergic Agents.” Drug Design and Discovery. 9 (3–4): 299–312. ISSN 1055-9612.
- Sprague, J. E., Johnson, M. P., Schmidt, C. J., Nichols, D. E. (October 25, 1996). “Mechanism of p-Chloroamphetamine Neurotoxicity.” Biochemical Pharmacology. 52 (8): 1271–1277. doi:10.1016/0006-2952(96)00482-0. ISSN 0006-2952.
- Cozzi, N. V., Frescas, S., Marona-Lewicka, D., Huang, X., Nichols, D. E. (March 1998). “Indan Analogs of Fenfluramine and Norfenfluramine.” Pharmacology, Biochemistry, and Behavior. 59 (3): 709–715. doi:10.1016/s0091-3057(97)00557-1. ISSN 0091-3057.
- Talaie, H.; Panahandeh, R.; Fayaznouri, M. R.; Asadi, Z.; Abdollahi, M. (2009). “Seizure Incidence with Tramadol.” Journal of Medical Toxicology. 5 (2): 63–67. doi:10.1007/BF03161089. eISSN 1937-6995. ISSN 1556-9039.
- Gillman, P. K. (2005). “Interaction of Monoamine Oxidase Inhibitors and Opioid Analgesics.” British Journal of Anaesthesia. 95 (4): 434–441. doi:10.1093/bja/aei210 Freely accessible. eISSN 1471-6771. ISSN 0007-0912. PMID 16051647.
- Anvisa (National Health Surveillance Agency). (2016). “Regulation on Psychoactive Substances.” [Online Document]. Retrieved from http://portal.anvisa.gov.br/documents/10181/3115436/%281%29RDC_130_2016_.pdf/fc7ea407-3ff5-4fc1-bcfe-2f37504d28b7
- Ministry of Food and Drug Safety (South Korea). (2018). “Controlled Substances Act.” [Online Document]. Retrieved from http://www.mfds.go.kr/eng/index.do
- Danish Medicines Agency. (September 2015). “Lists of Controlled Substances in Denmark.” [Online Document]. Retrieved from http://laegemiddelstyrelsen.dk/en/licensing/company-authorisations-and-registrations/euphoriant-substances/lists
- Bundesministerium der Justiz und für Verbraucherschutz (German Federal Ministry of Justice and Consumer Protection). “Controlled Substances Act.” [Online Document]. Retrieved from https://www.gesetze-im-internet.de/btmg_1981/index.html
- 厚生労働省 [Ministry of Health, Labour and Welfare (MHLW)]. (2022). “Designated Substances List” [PDF, in Japanese]. Retrieved from https://www.mhlw.go.jp/web/t_doc?dataId=00tb9309&dataType=0
- Bundeskanzlei [Federal Chancellery of Switzerland]. (2020). “Controlled Substances Regulation.” [Online Document]. Retrieved from https://www.admin.ch/opc/de/classified-compilation/20101220/index.html
- Başbakanlık Mevzuatı Geliştirme ve Yayın Genel Müdürlüğü (Republic of Turkey Prime Ministry Legislation Development and Publication Directorate). (2014). “Controlled Substances Act.” [PDF, in Turkish]. Retrieved from https://resmigazete.gov.tr/eskiler/2014/01/20140125-3-1.pdf
- United Kingdom Government. (2016). “Psychoactive Substances Act 2016.” [Online Document]. Retrieved from https://www.legislation.gov.uk/ukpga/2016/2/contents/enacted
