A critical review of Methedrone research chemical sellers reveals the complexities and potential risks associated with the online marketplace for designer drugs.
One of the primary concerns with Methedrone sellers is the lack of proper regulation and oversight in the online realm. These vendors often operate in a legal gray area, exploiting drug law gaps to distribute substances like Methedrone without adequate quality control or safety measures. This poses a significant danger to buyers who may unwittingly purchase impure, adulterated, or contaminated products, exposing themselves to severe health hazards.
Credibility is another considerable issue. Given the evolving nature of designer drugs, many Methedrone vendors may need more knowledge or expertise about the compounds they sell. This knowledge gap can lead to accurate product descriptions, proper dosage recommendations, and the spread of misinformation regarding the substance’s effects.
Moreover, some Methedrone sellers employ deceptive marketing tactics, labeling their products as “research chemicals” or “legal highs.” Such terminology can create a false sense of security for potential buyers. However, the legal status of these substances is often uncertain, potentially placing individuals in legal jeopardy.
Ethical concerns also come into play. Selling Methedrone and similar substances without providing comprehensive information on potential risks and responsible usage guidelines reflects a lack of responsibility toward consumers’ well-being.
Contents
- 1 Summary
- 2 Legal status
- 3 Discovery
- 4 Structure and reactivity
- 5 Availability
- 6 Mechanism of action
- 7 Metabolism
- 8 Side and dosage effects
- 9 Toxicity
- 10 Overdose
- 11 Effects on animals
- 12 Legality
- 13 FAQ
- 13.1 1. What is Methedrone?
- 13.2 2. What are the effects of Methedrone?
- 13.3 3. How is Methedrone administered?
- 13.4 4. Is Methedrone legal?
- 13.5 5. What is the dosage of Methadone?
- 13.6 6. What are the potential risks of Methedrone use?
- 13.7 7. Is Methedrone addictive?
- 13.8 8. Can Methedrone be detected in drug tests?
- 13.9 9. Are there any long-term health effects associated with Methedrone use?
- 13.10 10. Where can I find treatment or support for Methedrone addiction?
- 14 References
Summary
Mephedrone, also known as para-methoxymethcathinone, 4-methoxymethcathinone, bk-PMMA, PMMC, methoxyphedrine, or 4-MeOMC, belongs to the cathinone chemical class and is primarily used as a recreational drug. From a chemical standpoint, mephedrone shares close similarities with compounds such as para-methoxymethamphetamine (PMMA), methylone, and mephedrone.
Methedrone garnered significant media attention in 2009 following the tragic deaths of two young Swedish individuals. Toxicology analyses in both cases revealed that methedrone was the sole drug in their systems at the time of overdose and subsequent fatalities. These incidents underscored the potential dangers associated with the use of methedrone and brought it into the spotlight as a substance of concern in designer drugs.
| Identifiers | |
|---|---|
| show IUPAC name | |
| CAS Number | 530-54-1 |
| PubChem CID | 216281 |
| ChemSpider | 187475 |
| UNII | L7HY239I58 |
| ECHA InfoCard | 100.209.920 |
| Chemical and physical data | |
| Formula | C11H15NO2 |
| Molar mass | 193.246 g·mol−1 |
Legal status
| Legal status | DE: Anlage II (Authorized trade only, not prescriptible) UK: Class BI-P(Poland)[ |
|---|
Discovery
The production of methedrone was initially documented in 1933.
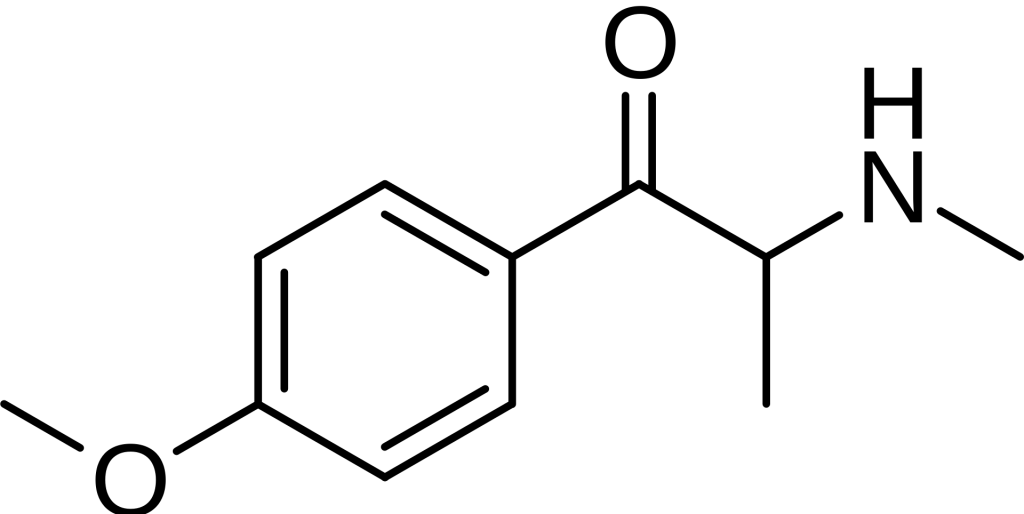
Structure and reactivity
Structure:
Methedrone is a synthetic cathinone closely related to the parent compound cathinone. It falls within the phenethylamine family due to the presence of a cyclic group of atoms represented as C6H5. Six carbons form a hexagonal ring in this structure, with each carbon bonded to five hydrogens. In contrast, the remaining carbon is connected to an atom or group of atoms other than hydrogen.[6][7][8]
Reactivity:
Limited information is available regarding the reactivity of methedrone, as articles or studies specifically need to address its chemical reactivity.
Synthesis:
The synthesis of mephedrone is outlined in Figure 1 and can be summarized in the following steps:
- Bromination of 1-(4-methoxyphenyl)propan-1-one to yield 2-bromo-1-(4-methoxyphenyl)propan-1-one.
- A reaction with methylamine converts 2-bromo-1-(4-methoxyphenyl)propan-1-one into 1-(4-methoxyphenyl)-2-(methylamino)propan-1-ol.
- The final step involves a reaction with potassium permanganate to complete the synthesis.
It’s worth noting that the synthesis of mephedrone, a structurally similar compound with only one fewer ether group than methadone, is well-documented and serves as a reference for the synthetic process.
Availability
Methadone is readily available for purchase in Europe (excluding Sweden) and in the majority of states in the US via online sources. It can also be found in brick-and-mortar stores such as head shops and retailers. Often, it is categorized alongside other novel or unregulated synthetic drugs and research chemicals, commonly marketed under the misleading label of “bath salts.”
Administration:
Methedrone is categorized as a research chemical with euphoric and stimulant properties that can be abused. It can be administered through various routes, including insufflation, ingestion, smoking, rectal, and intravenous methods. However, it is essential to note that methedrone differs significantly from substances like MDMA regarding both duration of effects and toxicity. Given the limited availability of medical literature on designer drugs, extreme caution should be exercised when using methedrone to mitigate potential risks and adverse effects.
Mechanism of action
Methedrone exhibits potent inhibition of the serotonin transporter (SERT) and norepinephrine transporter (NET), with relatively weak inhibition of the dopamine transporter (DAT). Clinically, methedrone’s DAT/SERT ratio falls below one, distinguishing it from other MDMA-like compounds. Methedrone does not favor inhibiting NET and DAT over SERT, unlike these compounds. This unique profile can lead to a potentially life-threatening adrenergic storm due to the lack of inhibition despite its tendency to release DAT. Methedrone stands out for its high selectivity for SERT among structurally similar compounds.
Mephedrone functions by inducing the release of norepinephrine (NE), dopamine (DA), and serotonin (5-HT) from cells preloaded with these monoamines. This mechanism categorizes it as a serotonin–norepinephrine–dopamine (SNDRA) releasing agent, also known as a triple releasing agent (TRA), a characteristic commonly associated with drugs of abuse.
Although mephedrone shares similarities with MDMA regarding interactions with monoamine transporters, in vivo studies have revealed a stronger hyperthermic reaction than what is typically observed with MDMA. Furthermore, methedrone’s impact on the serotonin transporter (SERT) can pose complications for individuals co-administering it with medications that affect serotonin levels, such as selective serotonin reuptake inhibitors (SSRIs). This interaction requires careful consideration and medical supervision to avoid adverse effects.
Metabolism
In examining the action of methedrone (4-MeOMC) within a biological system, it becomes crucial to investigate its stability in an aqueous solution. The rationale for this lies in the uncertainty of whether the compound will undergo enzymatic degradation or influence chemical mechanisms, such as pH and dissolved oxygen, when directly exposed to blood or urine. Researchers have conducted experiments to determine the compound’s half-life and the percentage remaining after 12 hours in buffers with varying pH levels, specifically at pH 4, 7, 10, and 12.
The findings reveal that, like most analogs, methedrone remains stable in acidic solutions. However, it undergoes decomposition in neutral and alkaline solutions, with the decomposition rate increasing as the solution becomes more essential.
In comparing methedrone to its analogs, several factors have been identified that affect their stability:
- The substituent group on the benzene ring.
- The groups attached to the nitrogen atom, although this factor does not apply to methedrone.
Methedrone and four other analogs feature substitutions in the meta- or para-positions. For these compounds, researchers have determined the rate constant, denoted as “k,” and constructed a Hammett plot by correlating the decomposition rate constant (0.693/half-life) at pH 12 with their Hammett constants obtained from the literature. This analysis reaffirms that methedrone is relatively stable even in essential solutions.
Furthermore, based on the literature findings, it is evident that a negative charge accumulates in the transition state during the rate-determining step. This deduction is supported by the strong linear correlation (r=0.9805) and the positive slope (calculated as 1.76).
There are two known possible metabolites for methedrone:
- Replacement of the carbon between the nitrogen group and R1 (an H-atom in the case of methedrone), resulting in a smaller compound.
- Replacement of the methyl group attached to the oxygen at the benzene group with an H-atom. This metabolite loses the epoxide group and transforms into a hydroxy compound.
Side and dosage effects
Dosage:
Information regarding the dosing of Methedrone is limited. According to user reports, a single dose of Methedrone typically ranges from 50 to 500 mg, and the effects can last anywhere from 45 minutes to two hours. It is essential to note that at least two documented fatal intoxications have been associated with Methedrone. In one instance, a postmortem femoral blood sample revealed a Methedrone concentration of 8.4 µg/g, and the patient passed away 16 hours after consumption, with concurrent use of other substances detected. In the second case, the concentration in the femoral blood sample was 9.6 µg/g, and Methedrone was the sole toxic compound detected. Scientific investigations of these cases suggest that Methedrone alone was responsible for these fatalities, and the concentrations in the femoral blood samples represented lethal levels of Methedrone. These studies highlight that the therapeutic index (or safety ratio) of Methedrone is low compared to other illicit drugs like Amphetamine. According to studies, a fatal dose is estimated to be around eight µg/g, while users typically exhibit doses ranging from 0.1 to 4.8 µg/g in their blood.
Side:
Anecdotal reports and case studies of human use of “bath salts,” including Methedrone, indicate that these substances induce powerful psychological effects. These psychological effects encompass psychotic behavior, paranoia, delusions, hallucinations, and even self-injury. Our knowledge of the physical effects of Methedrone in humans remains limited, but certain studies have explored its effects in animals, specifically mice. Research on the effects of Methedrone in mice has revealed a significant increase in behaviors such as circling, beam breaks, and hyperactivity. Additionally, mice exposed to Methedrone exhibited heightened salivation, head weaving, and stimulation. It is noteworthy that many jurisdictions consider Methedrone a legal drug. However, scientific investigations have shown it shares substantial pharmacological properties with banned substances such as mephedrone and methylone. Furthermore, the effects of Methedrone in mice closely resemble those of banned drugs. This suggests that Methedrone may carry similar risks and potential harm as commonly encountered illicit drugs.
Toxicity
Acute:
The existing research on the toxicity of methedrone is limited, with only one study conducted on mice[15]. Additionally, the lethal doses found in two Swedish methedrone-related fatalities have been compared to the methedrone concentrations in the blood of two other males who died under similar circumstances.
The average methedrone concentration in the blood of the deceased individuals was 1.3 μg/g blood, with one victim having an exceptionally high concentration of approximately 4.8 μg/g blood. The mean concentration in the blood of the deceased victims was 8.0 μg/g blood. Unfortunately, specific non-lethal and lethal dose thresholds remain unknown, indicating that the safety margin between a lethal and non-lethal dose of methedrone is likely very narrow. Consequently, the use of this drug is problematic, as poisonings and accidental fatalities can occur.
In a study assessing the effects of synthetic cathinones found in ‘bath salts,’ the impact of methedrone on mice was evaluated and compared to the effects of cocaine and methamphetamine. Various tests assessed motor coordination, balance, and overall behavioral effects. The mice did not exhibit differences in motor coordination or balance at administered doses of 10.0 mg/kg and 30.0 mg/kg. However, significant changes were observed in overall behavioral effects following mephedrone administration, including:
- Increased repetitive circular movement in mice.
- Hyperactivity.
- Excessive salivation.
- Increased head weaving.
- Heightened stimulation and tense body.
These effects indicate potential addiction potential[16][17]. Although not commonly reported in humans, excessive salivation is believed to result from methedrone’s influence on brain systems, primarily regulating autonomic responses.
Compared to mephedrone, methylenedioxypyrovalerone (MDPV), and 4-fluoro methcathinone (4-FMC), methedrone has a relatively slower onset. This slower onset heightens the risk because effects are not immediately evident, potentially leading to accidental overdose. It could also affect the drug’s popularity, as individuals tend to favor substances that cause rapid and pronounced initial increases in locomotor activity.
Chronic:
Chronic toxicity of methedrone has been poorly researched, with limited investigation. A post-mortem study offered some insights, revealing the presence of methedrone in the hair of deceased victims. However, no definitive conclusions were drawn from these findings.
No studies have examined the effects of methedrone use by pregnant females on the developing embryo, nor has its carcinogenic potential been thoroughly investigated.
Overdose
In 2009, two young men in southeast Sweden tragically died from methedrone overdose. Both individuals were discovered unconscious, and their conditions were critical. One of them experienced cardiorespiratory arrest en route to the hospital. At the same time, the second managed to survive for 16 hours under intensive care in the emergency department before succumbing to the effects of the overdose.
Effects on animals
Methedrone has been observed to impact the overall behavior of mice, resulting in several notable effects, including
- Excessive salivation
- Hyperactivity
- Increased head weaving
- Heightened repetitive circular movements in the mice
- Increased stimulation and heightened tension in the body
Legality
The sale of Methedrone was prohibited in Sweden as of December 9, 2009.
Furthermore, Methedrone has been classified as a controlled substance in China since October 1, 2015.
FAQ
1. What is Methedrone?
- Methedrone, also known as para-methoxymethcathinone (PMMC) and 4-methoxymethcathinone, is a recreational drug in the cathinone chemical class. It shares chemical similarities with compounds like mephedrone and methylone.
2. What are the effects of Methedrone?
- Methedrone’s effects can vary from person to person. Typically, it is known for inducing euphoria and stimulating properties, but it can also lead to side effects such as paranoia, hallucinations, and even self-injury in some cases.
3. How is Methedrone administered?
- Methedrone can be administered through various routes, including insufflation (snorting), ingestion (oral consumption), smoking, rectal administration, and intravenous injection. However, it’s essential to use caution due to its limited medical literature and potential risks.
4. Is Methedrone legal?
- The legality of Methedrone varies by jurisdiction. It is banned in some countries and regions due to its potential risks and harmful effects. Always check your local laws and regulations before considering its use.
5. What is the dosage of Methadone?
- Information about Methedrone dosages is limited. Users have reported doses ranging from 50 to 500 mg, with effects lasting between 45 minutes to two hours. However, there is a significant risk of overdose and toxicity, so caution is crucial.
6. What are the potential risks of Methedrone use?
- Methedrone use carries several risks, including the potential for overdose, severe psychological effects (paranoia, hallucinations), and physical side effects. There have been documented cases of fatal intoxications associated with Methedrone.
7. Is Methedrone addictive?
- Methedrone exhibits addictive potential, as it can lead to psychological dependence. Users should be aware of its addictive properties and take precautions.
8. Can Methedrone be detected in drug tests?
- Methedrone can be detected in some drug tests, but its detection window may vary depending on the specific test and the individual’s metabolism. It is essential to consider the potential legal consequences of using Methedrone.
9. Are there any long-term health effects associated with Methedrone use?
- Research on the long-term health effects of Methedrone is limited. Chronic toxicity and potential impacts on pregnancy have not been thoroughly studied, making it essential to exercise caution when considering its use.
10. Where can I find treatment or support for Methedrone addiction?
- If you or someone you know is struggling with Methedrone addiction, seeking professional help and support is crucial. Many addiction treatment centers and support groups are available to assist individuals in overcoming substance abuse issues.
References
- “Ustawa z dnia 15 kwietnia 2011 r. o zmianie ustawy o przeciwdziałaniu narkomanii (Dz.U. 2011 nr 105 poz. 614)”. Internetowy System Aktów Prawnych. Retrieved 17 June 2011.
- ^ “Cathinone and its analogues (e.g. mephedrone, methedrone, α- pyrrolidinovalerophenone)”. World Anti-Doping Agency. Archived from the original on 2014-11-06.
- ^ Jump up to:a b c d e f g h i Wikström M, Thelander G, Nyström I, Kronstrand R (November 2010). “Two fatal intoxications with the new designer drug methedrone (4-methoxymethcathinone)”. Journal of Analytical Toxicology. 34 (9): 594–8. doi:10.1093/jat/34.9.594. PMID 21073814.
- ^ “EMCDDA and Europol step up information collection on mephedrone”. Drugnet Europe. No. online 69. January–March 2010. Retrieved May 21, 2018.
- ^ Skita A (1933). “Eine neue Synthese von 1.2-Amino-ketonen”. Chemische Berichte. 66 (6): 858–866. doi:10.1002/cber.19330660615.
- ^ “EMCDDA – Synthetic cathinones profile (chemistry, effects, other names, synthesis, mode of use, pharmacology, medical use, control status)”. europa.eu.
- ^ “Technical Profile of Methedrone” (PDF). European Monitoring Centre for Drugs and Drug Addiction. Retrieved March 17, 2015.
- ^ Paillet-Loilier M, Cesbron A, Le Boisselier R, Bourgine J, Debruyne D (2014). “Emerging drugs of abuse: current perspectives on substituted cathinones”. Substance Abuse and Rehabilitation. 5: 37–52. doi:10.2147/SAR.S37257. PMC 4043811. PMID 24966713.
- ^ Schifano F, Albanese A, Fergus S, Stair JL, Deluca P, Corazza O, et al. (April 2011). “Mephedrone (4-methylmethcathinone; ‘meow meow’): chemical, pharmacological and clinical issues”. Psychopharmacology. 214 (3): 593–602. doi:10.1007/s00213-010-2070-x. hdl:2299/16594. PMID 21072502. S2CID 10529974.
- ^ DEA (Drug Enforcement Agency). Synthetic cathinones – DEA request for information posted 3/31/11; 2011 [retrieved 28.07.11].
- ^ Jump up to:a b Psychonaut Research Web Mapping Project, MDPV report, London, UK: Institute of Psychiatry, King’s College London; 2009.
- ^ Jump up to:a b c Yu H, Rothman RB, Dersch CM, Partilla JS, Rice KC (December 2000). “Uptake and release effects of diethylpropion and its metabolites with biogenic amine transporters”. Bioorganic & Medicinal Chemistry. 8 (12): 2689–92. doi:10.1016/S0968-0896(00)00210-8. PMID 11131159.
- ^ Jump up to:a b Tsujikawa K, Mikuma T, Kuwayama K, Miyaguchi H, Kanamori T, Iwata YT, Inoue H (July 2012). “Degradation pathways of 4-methylmethcathinone in alkaline solution and stability of methcathinone analogs in various pH solutions”. Forensic Science International. 220 (1–3): 103–10. doi:10.1016/j.forsciint.2012.02.005. PMID 22402273.
- ^ Mueller DM, Rentsch KM (February 2012). “Generation of metabolites by an automated online metabolism method using human liver microsomes with subsequent identification by LC-MS(n), and metabolism of 11 cathinones” (PDF). Analytical and Bioanalytical Chemistry. 402 (6): 2141–51. doi:10.1007/s00216-011-5678-8. PMID 22231510. S2CID 27846319.
- ^ Jump up to:a b c d e f Marusich JA, Grant KR, Blough BE, Wiley JL (October 2012). “Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice”. Neurotoxicology. 33 (5): 1305–13. doi:10.1016/j.neuro.2012.08.003. PMC 3475178. PMID 22922498.
- ^ Calabrese EJ (2008). “Addiction and dose response: the psychomotor stimulant theory of addiction reveals that hormetic dose responses are dominant”. Critical Reviews in Toxicology. 38 (7): 599–617. doi:10.1080/10408440802026315. PMID 18709568. S2CID 23303581.
- ^ Wise RA, Bozarth MA (October 1987). “A psychomotor stimulant theory of addiction”. Psychological Review. 94 (4): 469–92. CiteSeerX 10.1.1.471.1941. doi:10.1037/0033-295x.94.4.469. PMID 3317472.
- ^ Prosser JM, Nelson LS (March 2012). “The toxicology of bath salts: a review of synthetic cathinones”. Journal of Medical Toxicology. 8 (1): 33–42. doi:10.1007/s13181-011-0193-z. PMC 3550219. PMID 22108839.
- ^ Becker J, Neis P, Röhrich J, Zörntlein S (March 2003). “A fatal paramethoxymethamphetamine intoxication”. Legal Medicine. 5 (Suppl 1): S138-41. doi:10.1016/s1344-6223(02)00096-2. PMID 12935573.
- ^ “Two die of legal drug overdose”. The Local. Stockholm. 14 October 2009. Retrieved 21 May 2018.
- ^ “关于印发《非药用类麻醉药品和精神药品列管办法》的通知” (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 1 October 2015.