Beautiful Plants For Your Interior
Summary
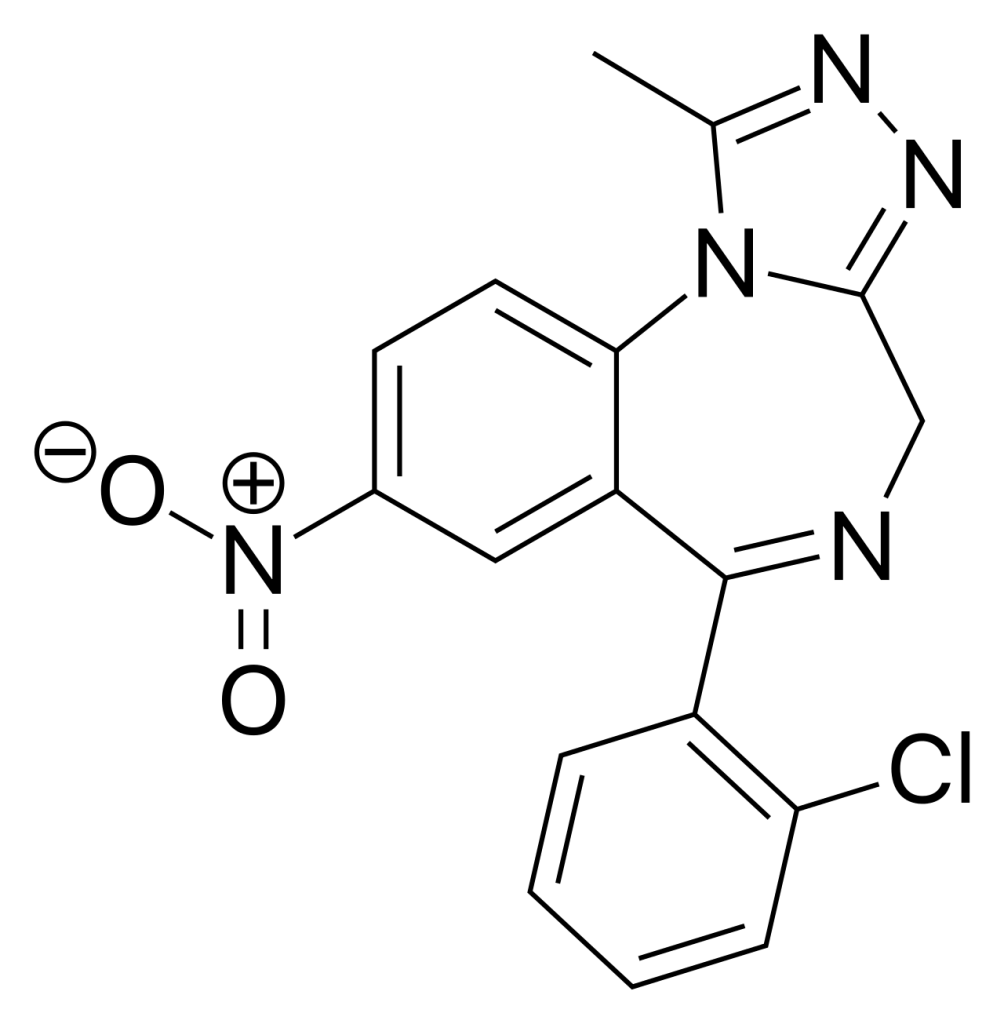
Clonazolam, also referred to as clonitrazolam, belongs to the triazolobenzodiazepine (TBZD) class, a category combining traditional benzodiazepines (BZDs) with a triazole ring. The existing knowledge about its effects and metabolic processes is limited, and clonazolam is available for purchase online as a designer drug.
The synthesis of clonazolam was first documented in 1971, and it was identified as the most potent compound within the series of substances tested.
It’s important to note that clonazolam carries a potentially higher risk compared to other designer benzodiazepines. Even at very low doses, as little as 0.5 mg, it can induce pronounced sedation and amnesia. Users should exercise caution and be aware of the potential effects and risks associated with this substance.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 33887-02-4 |
|---|---|
| PubChem CID | 12317881 |
| ChemSpider | 15468596 |
| UNII | HJH52YYC1X |
| CompTox Dashboard (EPA) | DTXSID301014166 |
| Chemical and physical data | |
| Formula | C17H12ClN5O2 |
| Molar mass | 353.77 g·mol−1 |
Legality
United Kingdom
In the United Kingdom, a legislative amendment in May 2017 to The Misuse of Drugs Act 1971 classified clonazolam as a Class C drug, along with several other designer benzodiazepines.
United States
Clonazolam is designated as a Schedule I controlled substance in the United States and has not received approval from the FDA for human consumption. Virginia State Law has precisely placed clonazolam, along with other medications like etizolam, flu alprazolam, flubromazolam, and flu bromazepam, into Schedule I status. In August 2020, Minnesota also categorized clonazolam as a Schedule I drug.
On December 23, 2022, the DEA initiated consideration regarding the temporary Schedule I status of Clonazolam. Subsequently, on July 25, 2023, the DEA published a pre-print notice that Clonazolam would be temporarily scheduled as a controlled substance from 07/26/2023 to 07/26/2025.
Australia
In Australia, clonazolam is classified under Schedule 9 according to federal law.
Sweden
Sweden’s public health agency proposed classifying clonazolam as a hazardous substance on June 1, 2015.
Effects
Clonazolam’s effects closely resemble those of other benzodiazepines, including anxiolysis, disinhibition, lethargy, muscle relaxation, and euphoria. However, it’s important to note that no dose of clonazolam is considered “safe” due to the lack of research and its extreme potency. Amounts exceeding 0.5 mg can lead to benzodiazepine overdose in specific individuals, resulting in symptoms like sedation, confusion, amnesia, impaired breathing, loss of consciousness, and even death. Furthermore, due to the rapid onset of dependence, withdrawal symptoms, including seizures and fatalities, may occur soon after the intoxication period. Users should be aware of these potential risks and exercise extreme cautio
FAQ
- What is Clonazolam?
- Clonazolam is a psychoactive drug classified as a designer benzodiazepine. It belongs to the triazolobenzodiazepine class, which combines the benzodiazepine structure with a triazole ring.
- Are Clonazolam’s effects similar to other benzodiazepines?
- Yes, Clonazolam’s effects are similar to those of traditional benzodiazepines, including anxiolysis (anxiety relief), disinhibition, sedation, muscle relaxation, and a sense of euphoria.
- Why is Clonazolam considered dangerous?
- Clonazolam is known for its extreme potency, and even small doses, such as 0.5 mg, can result in benzodiazepine overdose in some individuals. This can lead to symptoms like sedation, confusion, amnesia, impaired breathing, loss of consciousness, and, in severe cases, death.
- What are the legal regulations for Clonazolam?
- The legal status of Clonazolam varies by country and region. In the United States, it is classified as a Schedule I controlled substance at the federal level. Several U.S. states, like Virginia and Minnesota, have also scheduled it. The United Kingdom has classified it as a Class C drug, while Australia has placed it under Schedule 9.
- Is Clonazolam available for medical use?
- No, Clonazolam is not approved for medical use by the U.S. Food and Drug Administration (FDA) or similar regulatory bodies in other countries.
- What are the potential risks associated with Clonazolam use?
- Clonazolam is associated with a rapid onset of dependence. Abrupt discontinuation can lead to withdrawal symptoms, including seizures and fatalities. Due to the limited research on this substance, users should be aware of the potential health risks and exercise extreme caution.
- Why has Clonazolam been placed under temporary Schedule I status?
- In the United States, the Drug Enforcement Administration (DEA) announced the consideration of placing Clonazolam under temporary Schedule I status due to concerns about its safety and potential for abuse.
- How can I stay safe when dealing with substances like Clonazolam?
- The safest approach is to avoid using substances like Clonazolam altogether, especially given the lack of research and the associated risks. If you or someone you know is struggling with substance abuse or dependence, it’s essential to seek professional help and support.
- Is there ongoing research into Clonazolam?
- While research on Clonazolam is limited, ongoing studies are essential to understanding its effects, risks, and potential medical uses. Researchers aim to provide more insight into this substance’s pharmacology and safety.
- Where can I find reliable information and support regarding Clonazolam?
- It’s crucial to rely on trusted sources, such as healthcare professionals and government health agencies, for information on substances like Clonazolam. If you’re seeking assistance or support for substance use, numerous organizations and helplines are available to provide guidance and resources.
References
- Anvisa’s Controlled Substances List (2023-03-31)Anvisa, Brazil’s regulatory agency, issued RDC Nº 784, listing substances under special control. This includes substances with narcotic, psychotropic, and precursor properties. It was published in the Diário Oficial da União on April 4, 2023.
- Characterization of Designer Benzodiazepines (July 2015)Researchers characterized four designer benzodiazepines, including clonazolam, and identified their in vitro metabolites. The study aimed to gain insights into these compounds for forensic purposes.
- Human Urinary Metabolites of Designer Nitrobenzodiazepines (May 2016)This research identified the main human urinary metabolites of designer nitrobenzodiazepines, including clonazolam. High-resolution mass spectrometry was utilized for drug testing applications.
- Exploration of Metabolites of Synthetic Benzodiazepines (June 2016)The study explored the metabolites of eight synthetic benzodiazepines, shedding light on their potential metabolic pathways.
- Detectability of Designer Benzodiazepines (April 2017)Researchers examined the detectability of designer benzodiazepines, including clonazolam, using various immunochemical screening assays for drug testing.
- Blood Concentrations of Designer Benzodiazepines (November 2016)This research investigated the blood concentrations of new designer benzodiazepines in forensic cases, providing valuable data for toxicological analysis.
- Discovery of Clonazolam (November 1971)Clonazolam was first synthesized and described in this early research as one of the active compounds in the series tested, highlighting its central nervous system depressant activity.
- Designer Benzodiazepines – A New Challenge (June 2015)This publication addressed the emergence of designer benzodiazepines as a new challenge in the field of substance use and highlighted the need for research and regulation.
- Amendment to the Misuse of Drugs Act 1971 (2017)The UK’s Misuse of Drugs Act 1971 was amended in May 2017 to include clonazolam and several other designer benzodiazepines as Class C drugs.
- Legal Status in the United States (December 2022, July 2023)Clonazolam is classified as a Schedule I controlled substance in the United States, and its status was reviewed for temporary placement under Schedule I by the DEA in 2022 and 2023.
- Legal Status in Australia (February 2023)Clonazolam is categorized as Schedule 9 under federal law in Australia, highlighting its regulated status.
- Sweden’s Classification as a Hazardous Substance (June 2015)Sweden’s public health agency suggested classifying clonazolam as a hazardous substance in June 2015, reflecting growing concerns about its use.