Summary
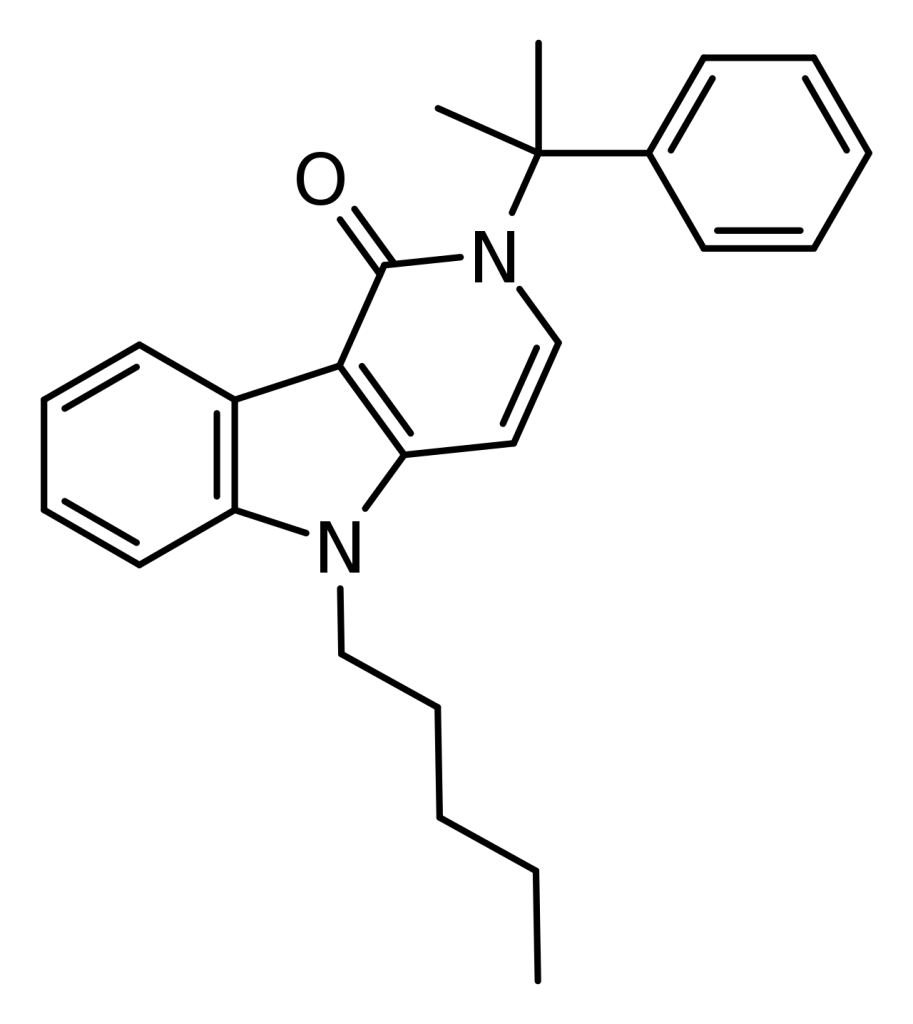
CUMYL-PEGACLONE, also known as SGT-151, is a synthetic cannabinoid rooted in the gamma-carboline structure and has been available as a designer drug. What sets CUMYL-PEGACLONE apart is its unique gamma-carboline core structure, which had not been observed in designer cannabinoids previously. However, it resembles other gamma-carboline cannabinoids disclosed by Bristol-Myers Squibb in 2001.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 2160555-55-3 |
|---|---|
| PubChem CID | 134818034 |
| ChemSpider | 68003813 |
| UNII | CUT2RV7EIQ |
| Chemical and physical data | |
| Formula | C25H28N2O |
| Molar mass | 372.5 g·mol−1 |
Legal status
Starting from May 4, 2023, there will be a 2-year period during which the DEA may initiate the filing process for permanent scheduling. If the DEA submits this filing on May 4, 2023, and does not complete the process for permanent placement, the temporary Schedule I order will conclude on May 4, 2025.
FAQ
1. What is CUMYL-PEGACLONE (SGT-151)?
CUMYL-PEGACLONE, also known as SGT-151, is a synthetic cannabinoid that has been available as a designer drug.
2. What is a synthetic cannabinoid?
Synthetic cannabinoids are man-made compounds designed to mimic the effects of natural cannabinoids found in cannabis, such as THC. They interact with the same receptors in the brain as natural cannabinoids.
3. How is CUMYL-PEGACLONE different from other synthetic cannabinoids?
CUMYL-PEGACLONE is distinct due to its gamma-carboline core structure. This structural feature sets it apart from other designer cannabinoids, although it shares some similarities with gamma-carboline cannabinoids disclosed by Bristol-Myers Squibb in 2001.
4. Is CUMYL-PEGACLONE legal and safe for use?
The legal status and safety of CUMYL-PEGACLONE can vary by region and country. Synthetic cannabinoids can have unpredictable and potentially harmful effects. Users should be aware of and adhere to local regulations.
5. What potential risks are associated with CUMYL-PEGACLONE use?
Using synthetic cannabinoids like CUMYL-PEGACLONE can pose various risks, including physical and mental health hazards, unknown long-term effects, and potential legal consequences. It is essential to exercise caution when dealing with these substances.
6. Are there any ongoing regulatory changes or scheduling considerations for CUMYL-PEGACLONE?
As of May 4, 2023, CUMYL-PEGACLONE may be subject to temporary scheduling by the DEA (Drug Enforcement Administration) for up to 2 years, during which the DEA may initiate the process for permanent scheduling. If no endless scheduling occurs, the temporary Schedule I order will expire on May 4, 2025.
7. Where can I find more information about CUMYL-PEGACLONE?
For further insights into CUMYL-PEGACLONE, it is advisable to consult scientific literature, research studies, and relevant authorities. Staying informed about its chemical properties, effects, and legal status is crucial for those interested or concerned about this substance.
References
- On July 24, 2023, Anvisa introduced “RDC Nº 804 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial” (Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control), shedding light on regulatory changes in Brazilian Portuguese. This information was published in Diário Oficial da União on July 25, 2023, and the original document can be accessed up to August 27, 2023.
- In August 2017, Ernst L, Brandhorst K, Papke U, Altrogge A, Zodel S, Langer N, and Beuerle T provided an update on the German situation regarding the identification and quantification of synthetic cannabinoids in ‘spice-like’ herbal mixtures. This research was documented in “Forensic Science International,” Volume 277, pages 51–58, with more details available through doi:10.1016/j.forsciint.2017.05.019. The PMID for this publication is 28601726.
- In July 2017, Angerer V, Mogler L, Steitz JP, Bisel P, Hess C, Schoeder CT, Müller CE, Huppertz LM, Westphal F, Schäper J, and Auwärter V delved into the structural characterization and pharmacological evaluation of the new synthetic cannabinoid CUMYL-PEGACLONE. This study was published in “Drug Testing and Analysis,” Volume 10, Issue 3, covering pages 597–603. For more in-depth information, refer to doi:10.1002/dta.2237. The PMID for this publication is 28670781.
- Mogler L, Wilde M, Huppertz LM, Weinfurtner G, Franz F, and Auwärter V explored the phase I metabolism of the synthetic cannabinoid CUMYL-PEGACLONE and its detection in human urine samples in January 2018. Their findings are available in “Drug Testing and Analysis,” Volume 10, Issue 5, from pages 886–891, and you can access further details through doi:10.1002/dta.2352. The PMID for this publication is 29314750.
- In February 2019, Halter S, Angerer V, Röhrich J, Groth O, Roider G, Hermanns-Clausen M, and Auwärter V explored Cumyl-PEGACLONE, a relatively safe synthetic cannabinoid receptor agonist entering the NPS market. This study was documented in “Drug Testing and Analysis,” Volume 11, Issue 2, spanning pages 347–349. To learn more, you can refer to doi:10.1002/dta.2545. The PMID for this publication is 30468574.
- Janssens L, Cannaert A, Connolly MJ, Liu H, and Stove CP conducted in vitro activity profiling of Cumyl-PEGACLONE variants at the CB1 receptor in September 2020. Their study provided insights into the effects of fluorination versus isomer exploration. This research can be explored in “Drug Testing and Analysis,” Volume 12, Issue 9, covering pages 1336–1343. For a comprehensive understanding, refer to doi:10.1002/dta.2870. The PMID for this publication is 32490586.
- Tiemensma M, Rutherford JD, Scott T, and Karch S examined the emergence of Cumyl-PEGACLONE-related fatalities in the Northern Territory of Australia in November 2020. This research was featured in “Forensic Science, Medicine, and Pathology,” Volume 17, Issue 1, from pages 3–9, and more information is available through doi:10.1007/s12024-020-00334-0. The PMID for this publication is 33185835.
- In a WO application from August 16, 2001, by Leftheris K, Zhao, R, Chen BC, and others, titled “Cannabinoid Receptor Modulators, Their Processes of Preparation, and Use of Cannabinoid Receptor Modulators in Treating Respiratory and Non-Respiratory Diseases,” Bristol-Myers Squibb Company revealed valuable information regarding cannabinoid receptor modulators and their applications.
- Wrobleski ST, Chen P, Hynes J, Lin S, Norris DJ, and others engaged in the rational design and synthesis of an orally active indolopyridone with anti-inflammatory properties in May 2003. Their work is documented in the “Journal of Medicinal Chemistry,” Volume 46, Issue 11, from pages 2110–2116, and can be explored further through doi:10.1021/jm020329q. The PMID for this publication is 12747783.
- Alam RM and Keating JJ reviewed the chemistry and pharmacology of newly emerging heterocyclic synthetic cannabinoid receptor agonists in March 2020. Their insights are available in “Drug Testing and Analysis,” Volume 12, Issue 3, from pages 297–315. To delve deeper into this review, you can refer to doi:10.1002/dta.2752. The PMID for this publication is 31854124.
- On January 18, 2019, “Sexton nya ämnen klassas som narkotika eller hälsofarlig vara” (in Swedish) was published by Folkhälsomyndigheten, addressing the classification of new substances as narcotics or hazardous goods.
- “Federal Register” holds additional information and documents regarding relevant regulations and schedules.