Contents
Summary
Desoxypipradrol, alternatively referred to as 2-diphenylmethylpiperidine (2-DPMP), is a pharmaceutical compound created by Ciba during the 1950s. It functions as a norepinephrine-dopamine reuptake inhibitor (NDRI).
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 519-74-4 5807-81-8 (HCl) |
|---|---|
| PubChem CID | 160506 |
| ChemSpider | 141045 |
| UNII | 49UNK1BV8T |
| CompTox Dashboard (EPA) | DTXSID90897502 |
| ECHA InfoCard | 100.007.525 |
| Chemical and physical data | |
| Formula | C18H21N |
| Molar mass | 251.373 g·mol−1 |
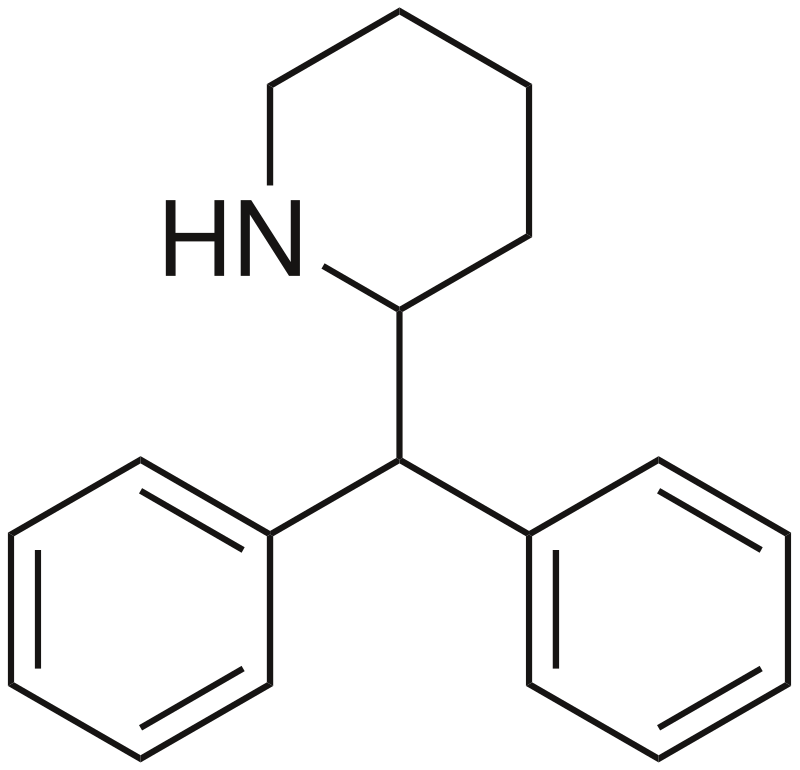
Chemistry
Desoxypipradrol shares a structural resemblance with two other compounds, methylphenidate and pipradrol, and all three exhibit a similar pharmacological effect. Among these piperidine compounds, desoxypipradrol stands out with the longest elimination half-life. This extended duration is attributed to its high lipophilicity and the absence of polar functional groups that are typically targeted by metabolic enzymes. In contrast, most psychostimulants have shorter durations of action. Methylphenidate, for instance, is short-acting due to the presence of a methyl-ester moiety that readily cleaves, forming a highly polar acid group. Pipradrol falls in between in terms of duration, possessing a hydroxyl group that can be conjugated (e.g., with glucuronide) to enhance its hydrophilicity and facilitate excretion, although it lacks easily metabolized groups.
History
In the 1950s, the pharmaceutical company CIBA, now known as Novartis, pioneered the development of Desoxypipradrol. It was explored for potential uses in addressing conditions like narcolepsy and ADHD. However, CIBA halted its development of Desoxypipradrol after creating the related drug methylphenidate. Methylphenidate was considered the superior option for ADHD treatment due to its shorter duration of action and more predictable pharmacokinetics. While Desoxypipradrol was investigated for other potential applications, such as aiding rapid recovery from anaesthesia, it did not progress further in development. On the other hand, a hydroxylated derivative known as pipradrol was introduced as a clinical drug, approved for treating depression narcolepsy and enhancing cognitive function in cases of organic dementia.
Detection in biological specimens
To confirm poisoning in hospitalized patients or support medicolegal death investigations, Desoxypipradrol levels can be measured in blood, plasma, or urine using liquid chromatography-mass spectrometry. In recreational users, one would typically find blood or plasma concentrations of Desoxypipradrol within the range of 10–50 μg/L. In cases of intoxication, these concentrations may exceed >100 μg/L, and in instances of acute overdosage, they can reach levels exceeding >600 μg/L.

Legal status
Desoxypipradrol’s structural resemblance to pipradrol has led to its classification as a controlled substance analogue in various countries, including Australia and New Zealand.
China: As of October 2015, 2-DPMP has been designated as a controlled substance in China.[6]
United Kingdom: On November 4, 2010, the UK Home Office initiated a prohibition on the importation of 2-DPMP based on a recommendation from the ACMD (Advisory Council on the Misuse of Drugs).[7]
Before this import ban, Desoxypipradrol was available in multiple products, notably “Ivory Wave,” marketed as a ‘legal high.’ Its usage resulted in numerous visits to the Emergency Department, prompting the UK government to request an assessment by the ACMD. There was even a case where a person had ingested nearly 1 gram of the drug, which could have been fatal without the administration of anaesthetic doses of benzodiazepines in the Accident and Emergency department.
The ACMD’s report highlighted serious harms associated with 2-DPMP, including prolonged agitation (lasting up to 5 days after drug use, sometimes severe enough to require physical restraint), paranoia, hallucinations, and myoclonus (muscle spasms/cramps).
Initially scheduled to become a class B drug on March 28, 2012,[10] the bill was revised due to the inclusion of two steroids deemed non-abusable. A new discussion on its status occurred on April 23, 2012, resulting in the decision to maintain the ban on 2-DPMP and expand it to encompass related chemicals.
Ultimately, Desoxypipradrol was classified as a class B drug and placed in Schedule I on June 13, 2012, with no recorded deaths associated with the drug during the period between the import ban and the possession ban. Additionally, “Esters and ethers of pipradrol” were subjected to control under the same amendment as class C drugs.
FAQ
1. What is Desoxypipradrol? Desoxypipradrol, also known as 2-DPMP, is a chemical compound with stimulant properties. It has structural similarities to other stimulants like methylphenidate and pipradrol.
2. What is the history of Desoxypipradrol? Desoxypipradrol was developed by the pharmaceutical company CIBA (now Novartis) in the 1950s. Initially, it was explored for potential medical applications, including the treatment of conditions like narcolepsy and ADHD.
3. Is Desoxypipradrol a controlled substance? Yes, Desoxypipradrol is classified as a controlled substance in various countries. For example, it is considered a controlled substance analogue in Australia and New Zealand. In the United Kingdom, it faced import bans and was eventually classified as a class B drug.
4. Why was Desoxypipradrol banned or controlled in some countries? Desoxypipradrol was banned or controlled due to concerns about its potential for abuse and the associated health risks. Reports of adverse effects, including prolonged agitation, paranoia, hallucinations, and muscle spasms, contributed to its classification as a controlled substance.
5. What are the expected effects of Desoxypipradrol use? Desoxypipradrol is a stimulant and is expected to produce effects similar to other stimulant drugs, such as increased alertness, energy, and focus. However, it is crucial to note that its use is associated with serious health risks.
6. Is Desoxypipradrol still available on the market? Desoxypipradrol is not commonly available on the legal market, especially in countries where it has been banned or classified as a controlled substance. Its availability may be restricted to certain research or forensic purposes.
7. What are the risks associated with Desoxypipradrol use? The use of Desoxypipradrol is associated with various risks, including cardiovascular issues, mental health disturbances, and potential addiction. It is essential to be aware of these risks and avoid recreational use.
8. Can Desoxypipradrol be detected in drug tests? Yes, Desoxypipradrol can be detected in blood, plasma, or urine using specific analytical methods, such as liquid chromatography-mass spectrometry. This detection can be used for medical diagnosis, poisoning confirmation, or forensic investigations.
9. Are there any recorded deaths related to Desoxypipradrol use? There were no recorded deaths associated with Desoxypipradrol use between the time of the import ban and the possession ban in the United Kingdom. However, its use has been linked to serious adverse effects.
10. Is Desoxypipradrol used for any legitimate medical purposes today? As of my last knowledge update in September 2021, Desoxypipradrol had limited or no recognized medical applications. Its use was primarily explored in research settings, but it was not approved for medical use. Always consult with healthcare professionals for the most up-to-date information on any substance.
References
- US Patent 2820038 – 2-Diphenyl-Methyl-Piperidine: This patent document likely pertains to the chemical compound 2-Diphenyl-Methyl-Piperidine, which is related to Desoxypipradrol and may contain information on its synthesis or applications.
- Ferris RM, Tang FL (September 1979): This research publication examines the effects of various isomers of amphetamine, methylphenidate, and deoxypipradrol on the uptake of neurotransmitters like norepinephrine and dopamine by synaptic vesicles in different regions of the rat brain.
- Tripod J, Sury E, Hoffmann K (June 1954): This study explores the analeptic (stimulant) effects of a new piperidine derivative, possibly an early investigation into compounds related to Desoxypipradrol.
- Bellucci G (June 1955): This research discusses drugs such as 2-Diphenylmethyl-piperidine hydrochloride and the methyl ester of 2-chloro-2-phenyl-2-(2-piperidyl)-acetic acid, which are known for their stimulating effects during anesthesia.
- Baselt RC (2014): This book, “Disposition of toxic drugs and chemicals in man,” contains information about Desoxypipradrol (2-DPMP) on pages 2172-2173 and is a resource for understanding the pharmacological aspects of the compound.
- “关于印发《非药用类麻醉药品和精神药品列管办法》的通知” (in Chinese): This Chinese document from the China Food and Drug Administration, dated 27 September 2015, likely pertains to regulations concerning the control and classification of psychoactive substances, possibly including Desoxypipradrol.
- Import ban on psychoactive drug UK Home Office: This refers to the UK Home Office’s decision to impose an import ban on psychoactive drugs, potentially including Desoxypipradrol.
- “ACMD advice on ‘Ivory Wave'”: This document from the UK Home Office, dated 27 January 2012, discusses the Advisory Council on the Misuse of Drugs (ACMD) advice regarding substances like Desoxypipradrol, particularly in relation to products like “Ivory Wave.”
- “The Misuse of Drugs Act 1971 (Amendment) Order 2012”: This UK Home Office document, dated 27 January 2012, likely details amendments to the Misuse of Drugs Act 1971, possibly related to the classification of substances like Desoxypipradrol.
- “Government accepts ACMD’s advice to schedule D2PM, 2-DPMP, and phenzepam”: This document, dated 27 January 2012, discusses the UK government’s acceptance of ACMD’s advice regarding the scheduling of substances, including Desoxypipradrol.
- “ACMD letter on further advice on the classification of two steroidal substances – February 2012”: This document from the UK Home Office, dated 14 February 2012, likely contains additional advice from the ACMD regarding the classification of specific substances, which may include Desoxypipradrol.
- “Draft Misuse of Drugs Act 1971 (Amendment) Order 2012”: This document from the UK Home Office, dated 23 April 2012, appears to be a draft of amendments to the Misuse of Drugs Act 1971, possibly including changes related to Desoxypipradrol.
- “A Change to the Misuse of Drugs Act 1971: control of pipradrol-related compounds and phenazepam”: Dated 7 June 2012, this document discusses changes to the Misuse of Drugs Act 1971 related to compounds like Desoxypipradrol, specifically mentioning the control of pipradrol-related substances.