Summary
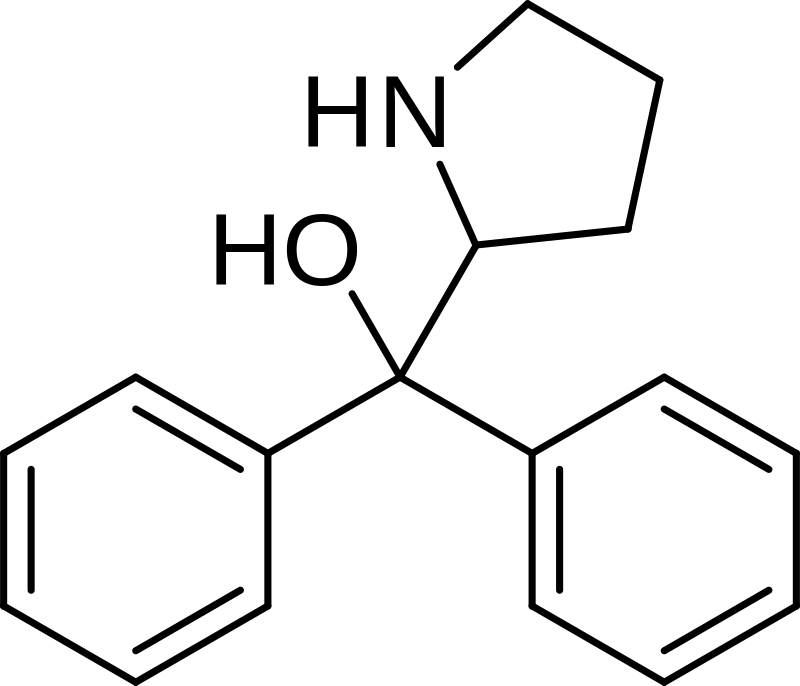
Diphenylprolinol (commonly known as D2PM) or (R/S)-(±)-diphenyl-2-pyrrolidinyl-methanol, is a substance categorized as a norepinephrine-dopamine reuptake inhibitor and has been utilized as a designer drug.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 22348-32-9 |
|---|---|
| PubChem CID | 204386 |
| ChemSpider | 177034 |
| UNII | 54U9UN7HN4 |
| CompTox Dashboard (EPA) | DTXSID60944996 |
| ECHA InfoCard | 100.118.791 |
| Chemical and physical data | |
| Formula | C17H19NO |
| Molar mass | 253.345 g·mol−1 |
Pharmacology
The more pharmacologically active enantiomer of diphenylprolinol is the dextrorotary (R)-(+)-enantiomer, although researchers have explored several related derivatives.
Side effects such as chest pain (which may indicate potential cardiovascular toxicity) have been reported in the recreational use of diphenylprolinol. However, it’s worth noting that diphenylprolinol was often combined with glaucine in party pill products, making it challenging to attribute these side effects to one specific drug definitively.
Other uses
Diphenylprolinol is employed in the preparation of the chiral CBS catalyst, which finds application in enantioselective organic synthesis.
FAQ
1. What is Diphenylprolinol?
Diphenylprolinol, also known as D2PM or (R/S)-(±)-diphenyl-2-pyrrolidinyl-methanol, is a chemical compound known for its norepinephrine-dopamine reuptake inhibitor properties. It has been used as a designer drug and has applications in organic synthesis.
2. What are the pharmacological properties of Diphenylprolinol?
Diphenylprolinol acts as a norepinephrine-dopamine reuptake inhibitor. The dextrorotary (R)-(+)-enantiomer is considered more pharmacologically active than its counterpart.
3. Are there any known side effects of Diphenylprolinol?
Recreational use of Diphenylprolinol has been associated with side effects, including chest pain, which may suggest potential cardiovascular toxicity. However, it’s important to note that such results may be confounded when Diphenylprolinol is combined with other substances, making it difficult to attribute specific side effects to this compound alone.
4. How is Diphenylprolinol used in organic synthesis?
Diphenylprolinol can be utilized to prepare the chiral CBS (Corey-Bakshi-Shibata) catalyst. This catalyst is employed in enantioselective organic synthesis, facilitating the production of chiral compounds with high optical purity.
5. Is Diphenylprolinol legal and regulated?
Regulations surrounding the legality of Diphenylprolinol vary by country and jurisdiction. It’s essential to consult local laws and regulations regarding its status and use.
6. What are the potential applications of Diphenylprolinol in research and industry?
Diphenylprolinol’s applications extend beyond its use as a designer drug. Its role in enantioselective organic synthesis makes it valuable in academic research and industrial processes where the creation of chiral molecules is required.
7. Are there any ongoing studies or developments related to Diphenylprolinol?
Research on Diphenylprolinol may continue to explore its pharmacological properties, potential medical applications, and synthesis techniques for chiral catalysts. Staying updated with scientific literature is advisable for the latest developments.
References
- Wood DM, Button J, Lidder S, Ovaska H, Ramsey J, Holt DW, Dargan P (June 2008). “Detection of the novel recreational drug diphenyl-2-pyrrolidinemethanol (D2PM) sold ‘legally’ in combination with glaucine”. Clinical Toxicology. 46 (5): 393.
- “Abstracts of the XXVIII International Congress of the European Association of Poison Centres and Clinical Toxicologists. May 6-9, 2008. Seville, Spain”. Clinical Toxicology. 46 (5): 351–421. June 2008. doi:10.1080/15563650802071703. PMID 18568796.
- US patent 5925666, Jackson PF, Slusher BS, “Pharmaceutical compositions and methods for treating compulsive disorders using pyrrolidine derivatives”, issued 20 July 1999, assigned to Eisai Corp of North America.
- Lidder S, Dargan P, Sexton M, Button J, Ramsey J, Holt D, Wood D (September 2008). “Cardiovascular toxicity associated with recreational use of diphenylprolinol (diphenyl-2-pyrrolidinemethanol [D2PM])”. Journal of Medical Toxicology. 4 (3): 167–169. doi:10.1007/bf03161195. PMC 3550040. PMID 18821489.
- Corey EJ, Bakshi RK, Shibata S (1987). “Highly enantioselective borane reduction of ketones catalyzed by chiral oxazaborolidines Mechanism and synthetic implications”. J. Am. Chem. Soc. 109 (18): 5551–5553. doi:10.1021/ja00252a056. ISSN 0002-7863.