Summary
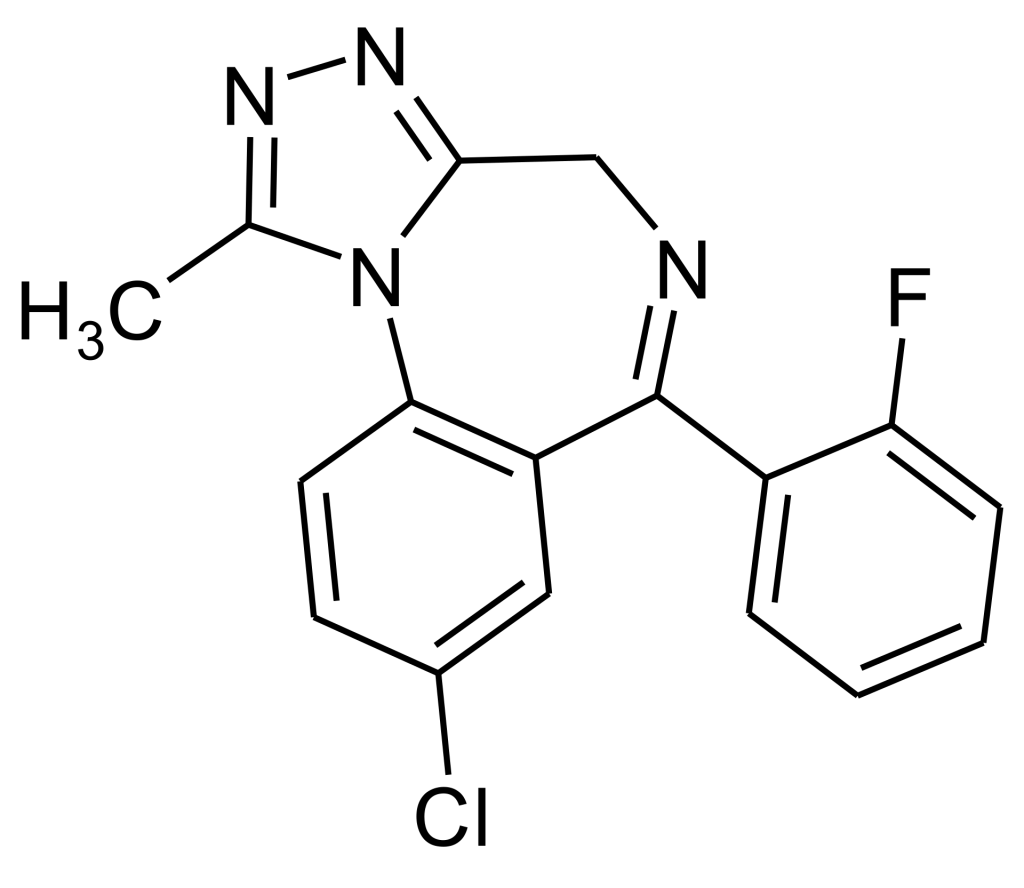
Flualprazolam is a tranquillizing agent belonging to the triazolobenzodiazepine (TBZD) class, characterized by benzodiazepines (BZDs) fused with a triazole ring. Its initial synthesis took place in 1976 but remained unmarked. This compound can be viewed as the triazole counterpart of fludiazepam and has gained popularity as a designer drug. It was officially identified as such in Sweden in 2018. Flualprazolam is recognized as the 2′-fluoro derivative of alprazolam or the fluorine-substituted analogue of triazolam, delivering comparable sedative and anxiolytic effects.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 28910-91-0 |
|---|---|
| PubChem CID | 10359044 |
| ChemSpider | 8534493 |
| UNII | FWF5L8D2BE |
| Chemical and physical data | |
| Formula | C17H12ClFN4 |
| Molar mass | 326.76 g·mol−1 |
Legal status
Flualprazolam is prohibited in Sweden and is also deemed illegal in the UK. Furthermore, in December 2019, the World Health Organization recommended the international scheduling of flu alprazolam as a Schedule IV medication under the Convention on Psychotropic Substances.
In the United States, both Oregon and Virginia have classified Flualprazolam as Schedule I. On December 23, 2022, the DEA initiated discussions on potentially assigning temporary Schedule I status to Flualprazolam. Subsequently, on July 25, 2023, the DEA issued a pre-print notice indicating that Flualprazolam would be temporarily scheduled as a controlled Schedule I substance from 07/26/2023 to 07/26/2025.
FAQ
- What is Flualprazolam?
- Flualprazolam is a tranquilizer belonging to the triazolobenzodiazepine (TBZD) class, which are benzodiazepines (BZDs) combined with a triazole ring. It is a chemical compound with sedative and anxiolytic effects, somewhat similar to other benzodiazepines.
- When was Flualprazolam first synthesized?
- Flualprazolam was initially synthesized in 1976, but it was never marketed as a pharmaceutical drug.
- Is Flualprazolam considered a designer drug?
- Yes, Flualprazolam has been sold as a designer drug, especially in recent years.
- When was Flualprazolam identified as a designer drug in Sweden?
- Flualprazolam was first definitively identified as a designer drug in Sweden in 2018.
- What is the chemical distinction between Flualprazolam and other similar benzodiazepines?
- Flualprazolam can be described as the 2′-fluoro derivative of alprazolam or the fluoro analogue of triazolam, replacing the chloro atom. This substitution results in similar sedative and anxiolytic effects.
- Is Flualprazolam legal in Sweden and the UK?
- No, Flualprazolam is banned in Sweden, and it is illegal in the UK.
- Has Flualprazolam been recommended for international scheduling by any regulatory body?
- Yes, in December 2019, the World Health Organization recommended flu alprazolam for international scheduling as a Schedule IV medication under the Convention on Psychotropic Substances.
- What is the legal status of Flualprazolam in the United States?
- Some states, like Oregon and Virginia, have placed Flualprazolam into Schedule I. In addition, there have been discussions about assigning temporary Schedule I status to Flualprazolam at the federal level by the DEA. A pre-print notice has been published indicating that Flualprazolam will become temporarily scheduled as a Schedule I controlled substance from 07/26/2023 to 07/26/2025.
References
- Anvisa’s Regulatory Update: Anvisa, the Brazilian Health Regulatory Agency, issued “RDC Nº 784 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial” on March 31, 2023. This resolution pertains to the classification and control of narcotic, psychotropic, precursor, and other substances, underscoring the significance of regulatory changes. It was published in the Diário Oficial da União on April 4, 2023.
- Historical Patent: A historical US patent, US 3987052, granted to Hester Jr JB on October 19, 1976, pertains to “6-Phenyl-4H-s-triazolo[4,3-a][1,4]benzodiazepines.” This patent provides insights into the early development of these chemical compounds.
- Toxicokinetics and Analytical Research: Several studies have delved into the toxicokinetics and analytical aspects of Flualprazolam. Research by Wagmann et al., published in July 2020, explores its metabolic fate, isozyme mapping, human plasma concentration, and primary urinary excretion products. Additionally, studies like those by Papsun et al. (March 2021), Rice et al. (April 2021), and Ling et al. (August 2022) contribute to our understanding of this substance’s analytical and toxicological properties.
- Legal Status in Sweden: Flualprazolam was banned in Sweden, as reported in June 2018. This legal decision signifies the regulatory measures taken by various countries regarding the substance.
- Postmortem Analysis: Studies have conducted postmortem analyses of femoral blood concentrations of Flualprazolam. For instance, Kriikku et al.’s research in February 2020 examined 33 postmortem cases, shedding light on the substance’s presence in fatal incidents.
- Quantitative Structure-Activity Relationship (QSAR) Model: Waters et al. (May 2018) have explored the use of a quantitative structure-activity relationship (QSAR) model to predict the binding of Flualprazolam to GABA-A receptors, contributing to our knowledge of its pharmacological effects.
- Designer Benzodiazepines: Flualprazolam falls within the category of designer benzodiazepines. Research by Moosmann and Auwärter (October 2018) and Chetraru et al. (February 2018) has provided insights into these emerging psychoactive substances, including Flualprazolam.
- Analytical Method Validation: Mei et al. (October 2019) validated an LC-MS/MS method for the quantification of 13 designer benzodiazepines in blood, including Flualprazolam. Such research is crucial for forensic and clinical purposes.
- Swedish Regulation: Information on the Swedish regulation “Förordning (1992:1554) om kontroll av narkotika” (Regulation on the Control of Narcotics) highlights the legal framework regarding controlled substances in Sweden.
- WHO Recommendation: In December 2019, the World Health Organization (WHO) recommended international scheduling for Flualprazolam as a Schedule IV medication under the Convention on Psychotropic Substances. This global perspective is significant for understanding its regulatory status.
- US DEA Temporary Scheduling: The United States, specifically the Drug Enforcement Administration (DEA), has considered placing Flualprazolam under temporary Schedule I status. On December 23, 2022, the DEA initiated this evaluation, and on July 25, 2023, a pre-print notice was published, indicating the substance’s temporary scheduling from 07/26/2023 to 07/26/2025.