Summary
HU-308, referred to as onternabez, PPP-003, and ARDS-003, represents a drug derived from cannabidiol (CBD) and functions as a potent cannabinoid agonist. Notably, HU-308 exhibits a remarkable degree of selectivity, primarily targeting the cannabinoid-2 receptor (CB2 receptor) subtype. Its selectivity for the CB2 receptor surpasses that of the CB1 receptor by over 5,000 times.
The synthesis and characterization of HU-308 were conducted in the laboratories of Raphael Mechoulam at the Hebrew University of Jerusalem during the late 1990s. HU-308, derived from pinene dimethoxy-DMH-CBD, was identified as a potent peripheral CB2-selective agonist through in vitro and animal studies, with initial findings dating back to 1990 and 1999.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 256934-39-1 |
|---|---|
| PubChem CID | 11553430 |
| ChemSpider | 8020425 |
| UNII | 8I5L034D55 |
| KEGG | D12305 |
| ChEBI | CHEBI:146244 |
| CompTox Dashboard (EPA) | DTXSID901010005 |
| Chemical and physical data | |
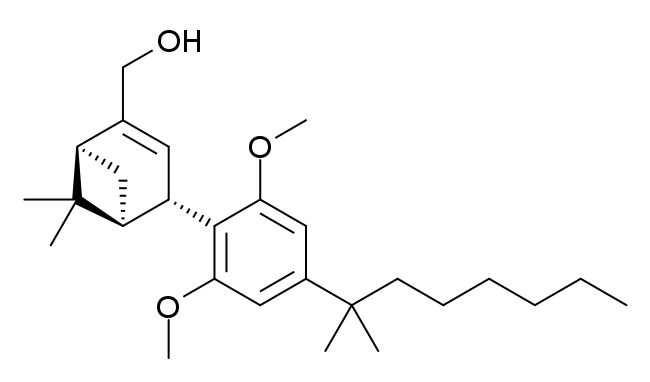
| Formula | C27H42O3 |
| Molar mass | 414.630 g·mol−1 |
Legal status
Tetra Bio-Pharma holds the intellectual property rights for HU-308, a non-psychoactive compound. While it is not federally scheduled in the United States, it is categorized as a Schedule I controlled substance in the state of Florida, making its purchase, sale, or possession illegal there.
FAQ
1. What is HU-308?
HU-308 is a non-psychoactive compound with potent cannabinoid agonist properties. It is known for its high selectivity for the cannabinoid-2 receptor (CB2 receptor).
2. Who owns the intellectual property rights to HU-308?
Tetra Bio-Pharma holds the intellectual property rights to HU-308.
3. Is HU-308 legal in the United States?
HU-308 is not scheduled at the federal level in the United States, meaning it is not a federally controlled substance. However, its legal status can vary by state. For example, in Florida, it is classified as a Schedule I controlled substance, making it illegal to buy, sell, or possess there.
4. What are the potential applications of HU-308?
HU-308’s non-psychoactive nature and selectivity for the CB2 receptor make it a subject of interest in various research and therapeutic applications. It may have potential in the development of medications targeting specific conditions.
5. How does HU-308 differ from other cannabinoids?
HU-308 is distinguished by its high selectivity for the CB2 receptor and its non-psychoactive properties. This sets it apart from cannabinoids that interact with the CB1 receptor, which can produce psychotropic effects.
6. Can individuals use HU-308 for recreational purposes?
HU-308 is primarily a research compound, and its use for recreational purposes may be subject to legal restrictions. It is essential to adhere to local laws and regulations regarding its use and possession.
7. Are there known side effects or adverse reactions associated with HU-308?
The specific side effects or adverse reactions of HU-308 may vary, and more research is needed to understand its safety profile fully. As with any substance, it’s crucial to use it under the guidance of a qualified healthcare professional when applicable.
8. What ongoing research is being conducted on HU-308?
Ongoing research aims to explore the potential therapeutic applications of HU-308, particularly its anti-inflammatory and non-psychoactive properties. Its unique characteristics make it a subject of interest in developing new medications.
9. How can one access HU-308 for research or medical purposes?
Access to HU-308 for research or medical purposes typically involves compliance with regulatory guidelines and may require appropriate licensing or authorization. Researchers and healthcare professionals should follow legal and ethical procedures for its acquisition and use.
10. What is the future outlook for HU-308 in cannabinoid research?
HU-308’s unique properties, including its non-psychoactive nature and selectivity for the CB2 receptor, position it as a promising candidate in cannabinoid research. Continued studies will likely reveal its full potential and applications in developing innovative therapies.
References
- Mechoulam R, Lander N, Breuer A, Zahalka J (1990): “Pioneering the way in tetrahydrocannabinol derivative synthesis.” This study marked the synthesis of the distinct enantiomers of a tetrahydrocannabinol derivative, a groundbreaking achievement in cannabinoid research.
- Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M (1999): “Discovering HU-308: Unveiling the CB(2) Receptor Agonist.” This research introduced HU-308 as a specific agonist for CB(2), a peripheral cannabinoid receptor, shedding light on its potential therapeutic applications.
- “Properties of HU-308: Unveiling the Formula C27H42O3.” The University of Pittsburgh’s Department of Chemistry delves into the chemical composition and properties of HU-308, contributing to a better understanding of this compound.
- US Patent 9549906 (2017): “Innovative Methods for Treating Ocular Inflammation & Pain.” This patent, assigned to Panag Pharma, Inc., explores the use of HU-308 and related compositions for treating ocular inflammation and pain, indicating its potential in the medical field.
- “Tetra Bio-Pharma’s Acquisition of Panag Pharma (2019).” Tetra Bio-Pharma’s acquisition of Panag Pharma showcases the growing interest and investment in HU-308 and related compounds for therapeutic purposes.
- “21 CFR — Schedules of Controlled Substances §1308.11 Schedule I.” A regulatory reference outlining the scheduling of controlled substances at the federal level, providing insights into the legal status of compounds like HU-308.
- “Florida Statutes Chapter 893 – Drug Abuse Prevention and Control.” Florida’s statutes on drug abuse prevention and control, which specify the legal classification of substances like HU-308 within the state.