Contents
Summary
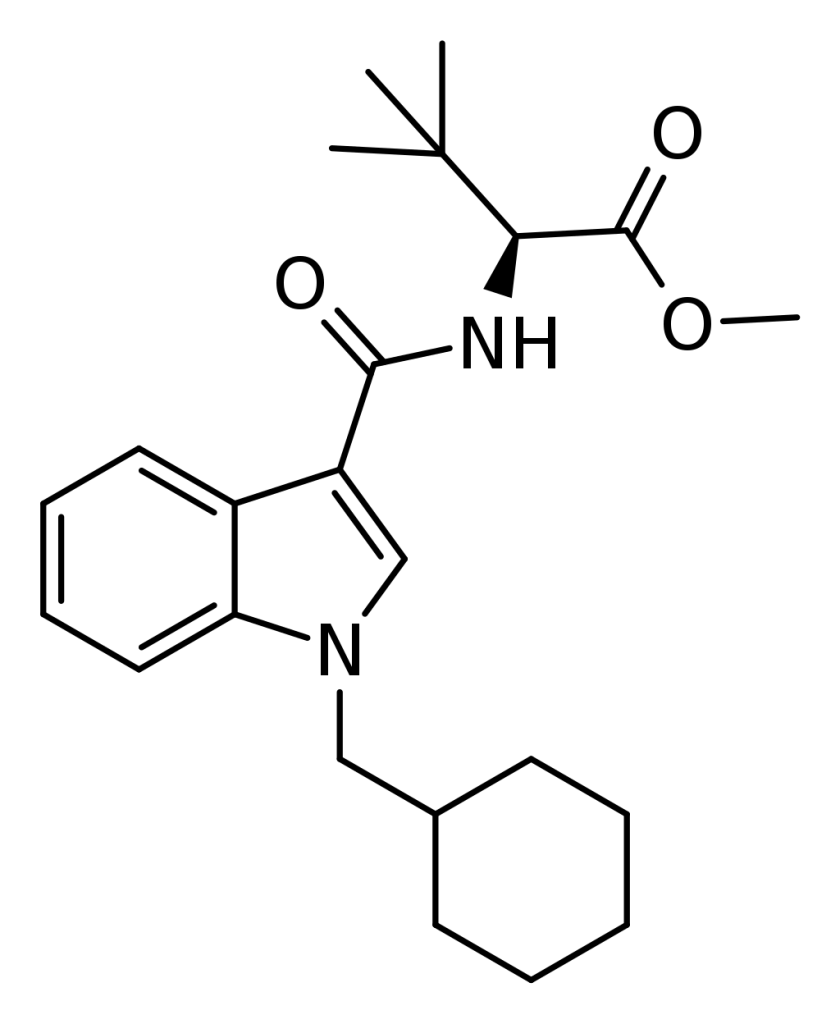
MDMB-CHMICA, classified as an indole-based synthetic cannabinoid, exhibits robust agonistic activity toward the CB1 receptor and has been commercialized online as a designer drug. Notably, while it was initially available under the name “MMB-CHMINACA,” the version corresponding to this codename (distinguished by the presence of isopropyl instead of t-butyl) emerged as AMB-CHMINACA in the designer drug market in 2015.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1971007-95-0 |
|---|---|
| PubChem CID | 125181404 |
| ChemSpider | 34450863 |
| UNII | X6JI7EA6EJ |
| Chemical and physical data | |
| Formula | C23H32N2O3 |
| Molar mass | 384.520 g·mol−1 |
Chemistry
Analysis of MDMB-CHMICA revealed that numerous commercial samples exclusively contained the (S)-enantiomer, as determined through vibrational and electronic circular dichroism spectroscopy and X-ray crystallography. This (S)-configuration within the tert-leucinate group is unsurprising, considering that MDMB-CHMICA is likely synthesized using the more readily available and cost-effective “L” form of the tert-leucinate reactant.
Pharmacology:
MDMB-CHMICA functions as a highly potent full agonist of the CB1 receptor, boasting an efficacy of 94% and an EC50 value of 0.14 nM. This EC50 value is notably lower than that of JWH-018 (1.13 nM) and AB-CHMINACA (0.27 nM).
Metabolism
The primary metabolic reactions of MDMB-CHMICA involve mono-hydroxylations and hydrolysis of the carboxylic ester function. In total, 31 distinct metabolites have been identified in vivo.
Side Effects
MDMB-CHMICA has been associated with seventy-one serious adverse events, including 42 acute intoxications and 29 fatalities across nine European countries between 2014 and 2016. These adverse events encompassed a wide range of symptoms, including unconsciousness, nausea, seizures, tachycardia, and severe behavioural disturbances, among others.
Legal Status
MDMB-CHMICA is classified as a Schedule I controlled substance in the United States. It is illegal in various countries and regions, including Austria, Canada, China, Croatia, Denmark, and more.
In August 2016, the European Commission proposed a ban on MDMB-CHMICA throughout the European Union. On 27 February 2017, the Commission implemented this ban, requiring Member States to institute control measures and criminal penalties by no later than 4 March 2018.
Seizures
Between 2014 and 2016, over 3,600 seizures of MDMB-CHMICA were reported in 19 European Union member states. One notable seizure included 40 kg of the substance in Luxembourg in December 2014.
FAQ
1. What is MDMB-CHMICA?
MDMB-CHMICA is a synthetic cannabinoid known for its potent activity on the CB1 receptor. It has been used as a designer drug and is associated with various health and legal concerns.
2. How is MDMB-CHMICA different from other synthetic cannabinoids?
MDMB-CHMICA is notable for its high potency as a full agonist of the CB1 receptor, which distinguishes it from many other synthetic cannabinoids.
3. What are the potential side effects of using MDMB-CHMICA?
The use of MDMB-CHMICA has been linked to a range of adverse effects, including unconsciousness, seizures, tachycardia, and even fatalities. The side effects can vary widely and may be severe.
4. Is MDMB-CHMICA legal?
The legal status of MDMB-CHMICA varies by country and region. It is classified as a Schedule I controlled substance in the United States and is illegal in several other countries. It’s essential to stay informed about the legal status in your area.
5. How is MDMB-CHMICA metabolized in the body?
MDMB-CHMICA undergoes metabolic processes that involve mono-hydroxylations and hydrolysis of the carboxylic ester function. Multiple metabolites have been identified in vivo.
6. What led to the ban of MDMB-CHMICA in the European Union?
In August 2016, the European Commission proposed a ban on MDMB-CHMICA due to concerns about its health and safety implications. This ban was implemented in February 2017, with Member States required to enforce control measures and penalties.
7. What should I do to stay safe regarding MDMB-CHMICA?
The best way to stay safe is to avoid using MDMB-CHMICA altogether. Due to its associated risks, it is strongly discouraged. If you or someone you know is experiencing adverse effects from its use, seek immediate medical attention.
8. Where can I find more information about MDMB-CHMICA?
Information about MDMB-CHMICA can be obtained from government health agencies, research studies, and educational resources dedicated to substance abuse prevention and awareness. It’s essential to rely on credible sources to understand the risks and consequences associated with this substance.
References
- The European Monitoring Centre for Drugs and Drug Addiction, in collaboration with the European Police Office, published a joint report on MDMB-CHMICA in July 2016. This informative report, available in PDF format, offers valuable insights into this synthetic cannabinoid.
- For further details on MDMB-CHMICA, you can refer to the information provided by Cayman Chemical, retrieved on 29th June 2015.
- Additional information on MDMB-CHMICA can be found from the Southern Association of Forensic Scientists, accessed on 29th June 2015.
- In a September 2016 study, Banister and his team explored the pharmacology of various synthetic cannabinoids, including MDMB-CHMICA, shedding light on their properties and effects. The research was published in ACS Chemical Neuroscience.
- Shevyrin et al. (August 2015) conducted an examination of synthetic cannabinoids with an indazole-3-carboxamide structure, furthering our understanding of compounds like MDMB-CHMICA.
- Andernach et al. (June 2016) delved into the absolute configuration of MDMB-CHMICA, providing insights into its chemical characteristics and presence in illicit products. This research was documented in Forensic Toxicology.
- Langer and his team (January 2016) contributed to the identification and quantification of synthetic cannabinoids, providing an update on the German situation in the spring of 2015.
- Maeda et al. (2018) investigated the effects of MDMB-CHMICA in conscious rats, shedding light on its impact on behavior and physiological responses. This study was published in Forensic Toxicology.
- Franz and his colleagues (2015) conducted research on the metabolism and urine analysis of MDMB-CHMICA, expanding our knowledge of its impact on the human body.
- Grigoryev and his team (July 2016) explored the human urinary metabolite pattern of MDMB-CHMICA, contributing to our understanding of its excretion and potential detection.
- Franz et al. (May 2017) delved into the phase I metabolism of MDMB-CHMICA, with a focus on detection in human urine samples. This study was published in Drug Testing and Analysis.
- The BBC reported on legal high confusion in Derby in April 2015, emphasizing the need for awareness regarding substances like MDMB-CHMICA.
- “TalkingDrugs” reported on the dangers of synthetic cannabinoid MMB-CHMINACA in July 2015, shedding light on the adverse effects and hospitalizations linked to its use.
- Westin and his team (February 2016) reported on a case of sudden cardiac death following the use of MDMB-CHMICA, highlighting the serious health risks associated with this synthetic cannabinoid.
- Adamowicz (April 2016) documented a fatal intoxication case involving MDMB-CHMICA, underlining the potentially life-threatening consequences of its use.
- Seywright et al. (September 2016) analyzed and reported clinical findings associated with the use of MDMB-CHMICA. This research was published in Clinical Toxicology.
- Hill and his team (September 2016) conducted a study on clinical toxicity arising from the use of synthetic cannabinoid MDMB-CHMICA, offering important insights into its health effects.
- A systematic review of adverse events linked to synthetic cannabinoids, including MDMB-CHMICA, was conducted by Tait et al. (January 2016), highlighting the need for comprehensive understanding and treatment of these substances.
- Meyyappan et al. (February 2017) reported on a case of poisoning due to MDMB-CHMICA, providing a crucial illustration of its toxic effects in Clinical Toxicology.
- Bäckberg and his team (March 2017) presented analytically confirmed intoxications involving MDMB-CHMICA from the STRIDA Project, contributing to our understanding of its real-world impact.
- The Drug Enforcement Administration (DEA) temporarily placed six synthetic cannabinoids, including MDMB-CHMICA, into Schedule I, a controlled substances category.
- China’s Food and Drug Administration issued a notification regarding the control of non-medical narcotic and psychoactive substances, impacting substances like MDMB-CHMICA.
- The legal status of MDMB-CHMICA was addressed by various entities, including state officials in the United States. The substance was banned in certain regions.
- In August 2016, the European Commission proposed a ban on MDMB-CHMICA across the European Union, reflecting concerns about its safety and impact.
- The European Commission implemented the ban on MDMB-CHMICA in February 2017, requiring member states to enforce control measures and penalties. This is in line with efforts to regulate the substance in the EU.
- The United Nations Office on Drugs and Crime provided a global SMART update in March 2015, offering insights into the global perspective on synthetic cannabinoids like MDMB-CHMICA.