Contents
Summary
Methoxetamine, often abbreviated as MXE, is a dissociative hallucinogenic substance that has been distributed as a designer drug. What sets it apart from many dissociatives, such as ketamine and phencyclidine (PCP), developed initially as pharmaceutical anesthetics, is that MXE was intentionally crafted to enhance the antidepressant effects of ketamine.
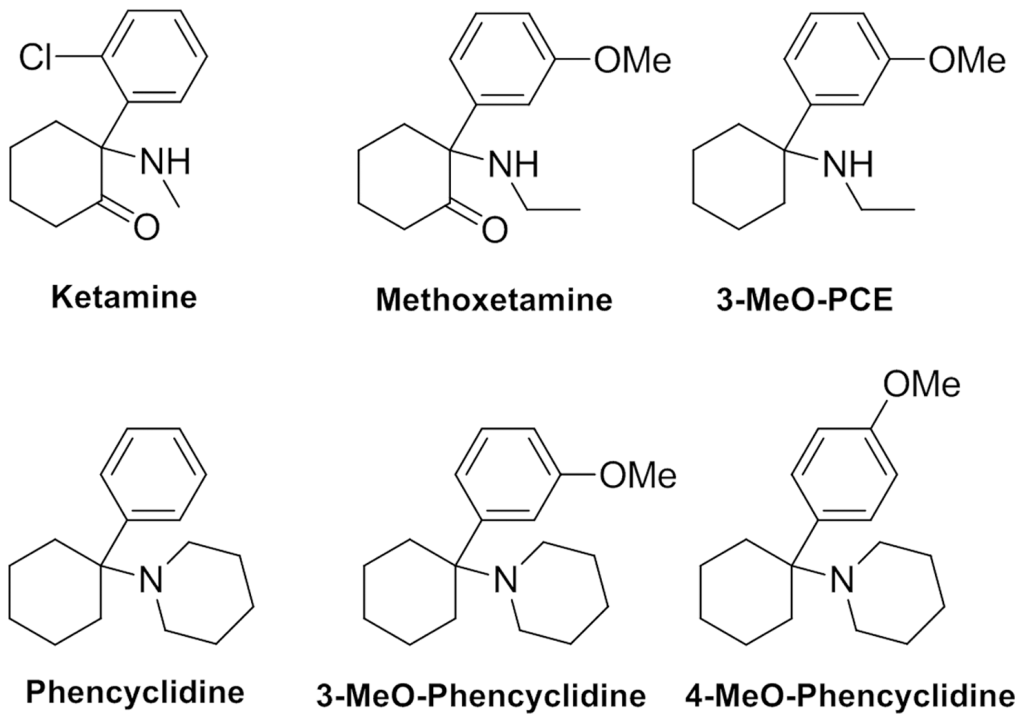
MXE belongs to the class of compounds known as arylcyclohexylamines. It primarily functions as an NMDA receptor antagonist, sharing this mechanism with other arylcyclohexylamines like ketamine and PCP.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1239943-76-0 |
|---|---|
| PubChem CID | 52911279 |
| ChemSpider | 24721792 |
| UNII | ZO5ZCE6E12 |
| CompTox Dashboard (EPA) | DTXSID301009327 |
| Chemical and physical data | |
| Formula | C15H21NO2 |
| Molar mass | 247.338 g·mol−1 |
Recreational use
Effects of Methoxetamine (MXE)
Methoxetamine, often encountered in powder form, elicits a range of effects that are akin to those of ketamine[1]. Initially, there was a misconception that MXE might possess opioid properties due to its structural resemblance to 3-HO-PCP. Still, scientific data do not support this assumption, as it demonstrates negligible affinity for the μ-opioid receptor.
Recreational use of MXE has been linked to instances of hospitalization, especially in cases of high or combined consumption, both in the United States and the United Kingdom. Notably, acute reversible cerebellar toxicity has been observed in individuals admitted to hospitals following MXE overdose, with this toxicity persisting for a duration of one to four days after exposure.
One of the purposes of developing MXE was to mitigate the urotoxicity associated with the abuse of ketamine. It was theorized that the increased potency and reduced dosage of MXE would lessen the accumulation of urotoxic metabolites in the bladder. However, similar to ketamine, MXE has been found to induce bladder inflammation and fibrosis after extensive, chronic administration in mice, though the dosages employed were considerably high. As of now, there are no documented reports of urotoxicity in humans within the medical literature.

Pharmacology
Methoxetamine, abbreviated as MXE, exhibits its pharmacodynamic profile through interactions with various receptors and transporters. Here are the critical details of MXE’s pharmacodynamics:
- NMDA Receptor (PCP Site): MXE primarily functions as a selective and high-affinity antagonist of the NMDA receptor, explicitly targeting the dizocilpine (MK-801) site, with a Ki of 257 nM. This action results in ketamine-like effects.
- Serotonin Transporter (SERT): MXE also acts as a serotonin reuptake inhibitor, with a Ki of 479 nM and an IC50 of 2,400 nM. This function influences the reuptake of serotonin, affecting mood and cognitive processes.
- Dopamine and Norepinephrine: In contrast, MXE has little to no effect on the reuptake of dopamine and norepinephrine, with Ki and IC50 values exceeding 10,000 nM. However, it can activate dopaminergic neurotransmission, particularly in the mesolimbic reward pathway, similar to other NMDA receptor antagonists like ketamine, PCP, and dizocilpine (MK-801).
MXE’s pharmacological characteristics are further supported by studies indicating that it may have rapidly-acting antidepressant effects akin to those observed with ketamine.
- Binding at Multiple Sites: A comprehensive assessment of MXE’s binding properties at 56 sites, including neurotransmitter receptors and transporters, revealed that MXE had Ki values greater than 10,000 nM for all sites except the dizocilpine site of the NMDA receptor and the serotonin transporter (SERT). This further underscores its selective actions in these areas.
In summary, MXE’s pharmacodynamics are characterized by its NMDA receptor antagonism, serotonin reuptake inhibition, and minimal impact on dopamine and norepinephrine reuptake, with a unique profile of binding at various sites in the central nervous system.
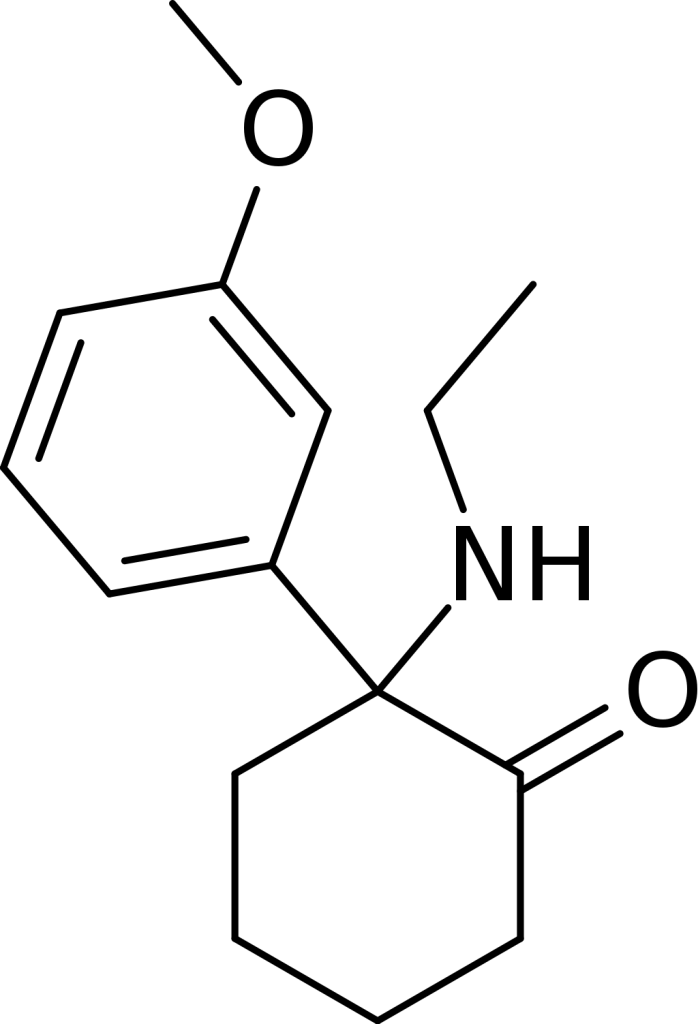
Chemistry
Methoxetamine (MXE): A Structural Perspective
MXE belongs to the class of arylcyclohexylamines and can be considered a derivative of eticyclidine (PCE). It can also be viewed as the β-Keto-derivative of 3-methoxyeticyclidine (3-MeO-PCE) or the N-ethyl homolog of methoxamine (MXM) and methoxpropamine (MXPr). Structurally, it shares a close relationship with ketamine and a more distant one with PCP.
When it comes to its solubility, MXE hydrochloride dissolves in ethanol, reaching concentrations of up to 10 mg/ml at 25°C.
Detection in Body Fluids
MXE has established a presence in the realm of forensics. A forensic standard for MXE is readily available, and the compound has been cataloged on the Forendex website, which serves as a resource for potential drugs of abuse. This facilitates its identification and monitoring in various forensic contexts.
History
The initial qualitative accounts of MXE’s effects started surfacing online in May 2010, marking the early steps of its emergence. In September 2010, MXE became commercially accessible, albeit on a limited scale. The compound’s popularity and distribution grew substantially within just a few months, leading to its formal recognition by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) in November. As of July 2011, the EMCDDA had pinpointed a total of 58 websites offering MXE for sale. It was available at prices ranging from 145 to 195 euros for a 10-gram quantity.
Society and culture
In January 2012, Mixmag reported that individuals in the dance music and clubbing community had bestowed upon MXE the colloquial moniker ‘roflcopter.’ Vice offered a comment, speculating that this term might only find favor among “the same politicians, parents, and journalists” who once referred to mephedrone as ‘meow meow.’ Following its reference as ‘mexxy’ in U.K. Home Office press releases, the media adopted this terminology.
A literature review was published in March 2012, delving into scientific literature and web-based information. It concluded that “the online availability of information on novel psychoactive drugs, such as MXE, may constitute a pressing public health challenge. Better international collaboration and innovative intervention strategies are necessary to address this rapidly evolving phenomenon”.
Legal Status
- Brazil: MXE was classified as a narcotic in Brazil in February 2014.
- Canada: As of January 2010, MXE is a controlled substance in Canada.
- China: As of October 2015, MXE is a controlled substance in China.
- European Union: On June 16, 2014, the European Commission proposed the banning of MXE across the European Union, subjecting violations to criminal sanctions.
- Israel: MXE became classified as an illegal narcotic in Israel in May 2012.
- Japan: MXE became a controlled substance in Japan starting on July 1, 2012, due to an amendment to the Pharmaceutical Affairs Law.
- Poland: MXE is a controlled substance (group II-P), making it illegal to produce, sell, or possess in The Republic of Poland as of July 1, 2015.
- Russia: MXE has been a controlled substance in Russia since October 2011.
- Sweden: MXE became classified as a narcotic in Sweden in late February 2012.
- Switzerland: MXE has been illegal in Switzerland since December 2011.
- United Kingdom: Prior to March 2012, MXE was not controlled by the U.K.’s Misuse of Drugs Act. However, in April 2012, it was placed under temporary class drug control, prohibiting its import and sale for 12 months. On October 18, 2012, the Advisory Council on the Misuse of Drugs released a report about MXE, classifying it as a Class B drug.
- United Nations: MXE was designated as a Schedule II drug in November 2016.
- United States: On June 6, 2022, the U.S. Drug Enforcement Administration published a final rule placing MXE in Schedule I of the Controlled Substances Act. Additionally, some states within the U.S., including Alabama, Florida, and Utah, have classified MXE as a controlled substance, rendering it illegal to buy, sell, or possess in these regions.
FAQ
- What is Methoxetamine (MXE)?
- Methoxetamine, often referred to as MXE, is a dissociative hallucinogen that belongs to the arylcyclohexylamine class of compounds. It is chemically related to drugs like ketamine and phencyclidine (PCP).
- How does MXE differ from ketamine and PCP?
- MXE was intentionally designed to enhance the antidepressant effects of ketamine. While it shares some similarities with ketamine and PCP in its products, it was developed with specific modifications to increase its antidepressant properties.
- What are the effects of MXE?
- MXE is reported to produce effects similar to ketamine, including dissociation, hallucinations, and altered perception. It was initially believed to have opioid-like properties, but the research did not support this assumption. High or combined consumption of MXE has been linked to hospitalizations in some cases.
- Are there any health risks associated with MXE use?
- Yes, there are health risks associated with MXE use. Acute reversible cerebellar toxicity has been documented in cases of MXE overdose. While it was developed to mitigate the urotoxicity related to ketamine, high doses of MXE have also been found to produce bladder inflammation and fibrosis in animal studies.
- What is the legal status of MXE in different countries?
- The legal status of MXE varies from country to country. As of 2022, MXE is controlled or banned in many nations, including the United States, Canada, the United Kingdom, European Union countries, Japan, Israel, Russia, Sweden, Switzerland, and more. It is essential to check the specific laws in your region regarding the possession and use of MXE.
- When did MXE first appear in the media and as a commercially available substance?
- The qualitative effects of MXE were first described online in May 2010, and it became commercially available on a small scale in September 2010. By July 2011, the European Monitoring Centre for Drugs and Drug Addiction had identified multiple websites selling the compound.
- What are the street names for MXE?
- In the dance music and clubbing community, MXE has been given the slang name ‘roflcoptr.’ It has also been referred to as ‘mexxy’ in some media outlets.
- Is there a forensic standard available for MXE?
- Yes, a forensic standard of MXE is available, and the compound has been listed on the Forendex website as a potential drug of abuse.
References
- In a study conducted by Kjellgren A and Jonsson K in 2013, they delved into the experiences induced by the “legal high” known as Methoxetamine (MXE), which was obtained from the internet. The findings of this phenomenological investigation were published in the Journal of Psychoactive Drugs (2013, 45(3), 276–286). This research provided valuable insights into the effects of MXE on users.
- The Federal Register (FederalRegister.gov) released a significant announcement on June 21, 2022, which is a crucial reference for the regulatory landscape concerning various substances and compounds.
- Anvisa, the Brazilian Health Regulatory Agency, issued Collegiate Board Resolution No. 804 on July 24, 2023. This resolution, published in the Diário Oficial da União on July 25, 2023, outlined lists of substances falling under special control, including narcotic and psychotropic compounds.
- The European Monitoring Centre for Drugs and Drug Addiction presented its Annual Report in 2010, offering valuable insights into the drug landscape. This report, which was archived from the original on March 14, 2012, contained crucial information for understanding drug-related trends.
- Morris H and Wallach J published a comprehensive review in 2014 that explored the non-medical use of dissociative drugs, such as PCP and MXE. This enlightening review, found in Drug Testing and Analysis (2014, 6(7–8), 614–632), provided a thorough examination of these substances.
- In a captivating interview documented by Vice Magazine in 2011, a ketamine chemist shared insights into the world of arylcyclohexylamine chemistry, shedding light on the synthesis of these compounds. This interview, although archived from the original on January 30, 2012, offered a unique perspective.
- Roth BL, Gibbons S, Arunotayanun W, Huang XP, Setola V, Treble R, and Iversen L conducted research in 2013, uncovering the affinity and selectivity of the ketamine analogue Methoxetamine for the glutamate NMDA receptor. Their findings were published in PLOS ONE (2013, 8(3), e59334), offering valuable data for the field.
- Wood DM, Davies S, Puchnarewicz M, Johnston A, and Dargan PI, in a study conducted in May 2012, investigated the acute toxicity associated with Methoxetamine, a ketamine derivative used recreationally. The results, found in the European Journal of Clinical Pharmacology (2012, 68(5), 853–856), provided essential information on the risks associated with this substance.
- Three cases of Methoxetamine-associated reversible cerebellar toxicity, confirmed through analytical means, were documented in a study by Shields JE, Dargan PI, Wood DM, Puchnarewicz M, Davies S, and Waring WS in June 2012. This investigation, available in Clinical Toxicology (2012, 50(5), 438–440), emphasized the importance of understanding the adverse effects of this substance.
- In April 2014, a pair was hospitalized after consuming the designer drug known as Mexxy. This incident, as reported by BBC News, highlighted the potential dangers associated with novel psychoactive substances.
- Dargan PI, Tang HC, Liang W, Wood DM, and Yew DT conducted a study in March 2014, demonstrating significant bladder and renal toxicity in mice after three months of Methoxetamine administration. Their findings, documented in Clinical Toxicology (2014, 52(3), 176–180), raised concerns about the potential harm of this substance.
- Roth BL and Driscol J developed the PDSP Ki Database, which is a valuable resource for understanding psychoactive substances and their interactions with receptors. This database, maintained by the University of North Carolina at Chapel Hill and the United States National Institute of Mental Health, is a pivotal reference for researchers.
- Hondebrink L, Kasteel EE, Tukker AM, Wijnolts FM, Verboven AH, and Westerink RH conducted a neuropharmacological characterization of Methoxetamine in September 2017. Their study, published in Neuropharmacology (2017, 123, 1–9), deepened our understanding of this new psychoactive substance.
- Zanda MT, Fadda P, Chiamulera C, Fratta W, and Fattore L presented a review of case reports and preclinical findings related to Methoxetamine in September 2016. Their work, available in Behavioural Pharmacology (2016, 27(6), 489–496), shed light on the serious adverse effects of this substance.
- Hofer KE, Grager B, Müller DM, Rauber-Lüthy C, Kupferschmidt H, Rentsch KM, and Ceschi A reported Ketamine-like effects after recreational use of Methoxetamine in July 2012. Their findings, published in Annals of Emergency Medicine (2012, 60(1), 97–99), highlighted the potential risks associated with this compound.
- Methoxetamine was found to produce rapid and sustained antidepressant effects, possibly through glutamatergic and serotonergic mechanisms, according to a study by Botanas CJ, Bryan de la Peña J, Custodio RJ, Joy Dela Peña I, Kim M, and Woo T, published in Neuropharmacology (2017, 126, 121–127).
- Horsley RR, Lhotkova E, Hajkova K, Jurasek B, Kuchar M, and Palenicek T conducted a detailed pharmacological evaluation of Methoxetamine (MXE) in the Wistar rat in September 2016. Their comprehensive research, available in Brain Research Bulletin (2016, 126(Pt 1), 102–110), provided a thorough examination of the substance’s effects.
- The safety data sheet for Methoxamine (hydrochloride) was provided by Cayman Chemicals and offered crucial safety information for handling this compound.
- The Southern Association of Forensic Scientists also featured information about Methoxetamine, contributing to the broader understanding of this substance.
- In November 2011, the European Monitoring Centre for Drugs and Drug Addiction released a report summarizing the results of online sales of new psychoactive substances, also known as “legal highs.” This report, available as a PDF, was archived from the original on February 2, 2012, and it offered insights into the online sale of such substances.
- An article in Mixmag (January 18, 2012) explored the legal and safety aspects of Methoxetamine (MXE), describing it as a new chemical analogue of ketamine, and provided a critical perspective on its use.
- Vice Magazine conducted an interview in 2011 with the inventor of Roflcoptr, a new drug that had garnered significant attention in Britain. This interview, although archived from the original on January 25, 2012, offered insights into the emergence of novel psychoactive substances.
- In March 2012, The Guardian reported on the banning of the “legal high” drug Mexxy under new government powers, shedding light on the regulatory measures being taken to address these substances.
- Corazza O, Schifano F, Simonato P, Fergus S, Assi S, Stair J, and others examined the phenomenon of new drugs on the internet, with a focus on the ketamine derivative Methoxetamine, in March 2012. Their findings, documented in Human Psychopharmacology (2012, 27(2), 145–149), emphasized the challenges posed by the online availability of such substances.
- Anvisa, in Portuguese, included 21 substances in its list of prohibited drugs, underscoring the regulatory efforts to control potentially harmful compounds.
- A document titled “Status Decision Of Controlled And Non-Controlled Substance(s)” was issued on January 31, 2011, offering insights into the legal classification of various substances.
- The China Food and Drug Administration released a notification in Chinese on September 27, 2015, concerning the control of non-medicinal narcotic and psychotropic substances, outlining their regulatory measures.
- The European Commission took decisive action in June 2014, issuing an EU-wide ban on four new substances, further emphasizing the global efforts to control novel psychoactive substances.
- The Israeli Ministry of Health, in Hebrew, issued regulations concerning the classification of dangerous substances, demonstrating their commitment to regulating potentially harmful compounds.
- The Russian Government, in a resolution dated October 6, 2011, made decisions regarding the control of various substances, emphasizing the international efforts to monitor and regulate these compounds.
- The Swedish authorities classified six new substances as narcotics, highlighting their commitment to addressing the risks associated with emerging psychoactive substances.
- In Germany, an ordinance outlined lists of narcotic drugs, psychotropic substances, precursors, and auxiliary chemicals, underscoring the regulatory framework in place for these compounds.
- The Independent reported a health alert in February 2012 concerning a substance marketed as “safe ketamine.” This alert emphasized the importance of public health and safety in regulating novel psychoactive substances.
- The UK government took action to address the issue of “safe ketamine” and referred the matter to drug experts, signaling their commitment to ensuring the safety of the population.
- A statement of evidence regarding Methoxetamine was released in February 2012, providing critical insights into the risks and effects associated with this substance.
- The UK Home Office provided information on Methoxetamine, offering valuable data for those seeking to understand the regulatory stance on this compound.
- The Home Secretary of the UK responded to the advice of the Advisory Council on the Misuse of Drugs (ACMD) regarding Methoxetamine, indicating the government’s intention to regulate this substance.
- In 2013, the UK Home Office issued a circular (004-2013) outlining updates and regulations related to various substances, including Methoxetamine.
- The United Nations Office on Drugs and Crime (UNODC) announced that the Commission on Narcotic Drugs had made decisions on international control of PMMA, α-PVP, 4,4′-DMAR, MXE, and Phenazepam, underscoring the global commitment to addressing the risks associated with these substances.
- In June 2022, the Federal Register announced the placement of Methoxetamine (MXE) in Schedule I, highlighting its legal classification in the United States.
- The Alabama State Board of Health outlined controlled substances in Chapter 420-7-2, providing essential information for those involved in the regulation of such compounds.
- Florida statutes pertaining to controlled substances were available online, offering critical legal information regarding the classification of various substances.
- Utah Code 58-37-4.2 listed controlled substances, providing a reference for substances subject to regulation in Utah.