Summary
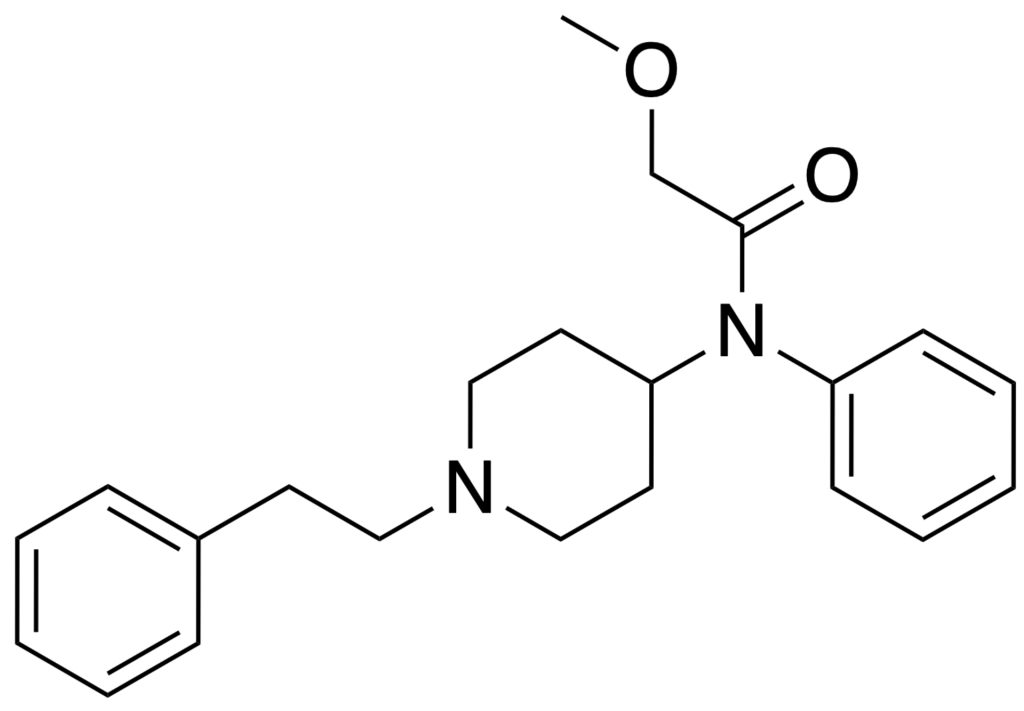
Methoxyacetylfentanyl, often referred to as MAF, is an opioid analgesic. It is structurally related to fentanyl and has been available for purchase on the internet as a designer drug.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 101345-67-9 |
|---|---|
| PubChem CID | 968688 |
| ChemSpider | 838859 |
| UNII | U7IAB3Z3V4 |
| ChEMBL | ChEMBL1740467 |
| Chemical and physical data | |
| Formula | C22H28N2O2 |
| Molar mass | 352.47 g·mol−1 |
Side effects
The side effects of fentanyl analogues closely mirror those of fentanyl itself, encompassing symptoms such as itching, nausea, and potentially severe respiratory depression, which can be life-threatening. Fentanyl analogues have caused numerous fatalities across Europe and the former Soviet republics, with a resurgence in use notably starting in Estonia in the early 2000s. Novel derivatives of these analogues continue to emerge.
A new wave of fentanyl analogues and the associated fatalities began around 2014 in the United States and has steadily increased in prevalence. Especially since 2016, these substances have been responsible for hundreds of overdose deaths each week.
Legal Status:
Methoxyacetylfentanyl was classified as a Schedule I substance in the United States in October 2017. This scheduling was implemented to prevent an imminent hazard to public safety.
FAQ
1. What is Methoxyacetylfentanyl (MAF)?
Methoxyacetylfentanyl, often referred to as MAF, is an opioid analgesic that is structurally related to fentanyl. It has been available for purchase on the internet as a designer drug.
2. What are the side effects associated with MAF and fentanyl analogues?
The side effects of MAF and fentanyl analogues closely resemble those of fentanyl itself. These may include itching, nausea, and potentially severe respiratory depression, which can be life-threatening.
3. What is the history of fentanyl analogues and their impact on public health?
Fentanyl analogues have caused numerous fatalities in Europe, the former Soviet republics, and the United States. A resurgence in use began in Estonia in the early 2000s, and novel derivatives of these analogues continue to appear. A new wave of fentanyl analogues and associated fatalities started around 2014 in the United States and has steadily increased in prevalence.
4. What is the legal status of Methoxyacetylfentanyl in the United States?
Methoxyacetylfentanyl was classified as a Schedule I substance in the United States in October 2017. This scheduling was done to prevent an imminent hazard to public safety.
5. Are fentanyl analogues safe for use or prescribed for medical purposes?
No, fentanyl analogues like MAF are not approved for medical use and are not prescribed by healthcare professionals. They are considered designer drugs and are not intended for legitimate medical applications.
6. Where can I find more information about the risks associated with MAF and fentanyl analogues?
Seek information from reputable sources such as government health agencies, addiction treatment centres, and substance abuse hotlines. It is advisable to consult with a healthcare professional if you have concerns about MAF or similar substances and their effects.
References
- European Monitoring Centre for Drugs and Drug Addiction; European Union Agency for Law Enforcement Cooperation (February 2017). “Exploring the Enigma: Methoxyacetylfentanyl.” This collaborative report delves into a novel psychoactive substance known as 2-methoxy-N-phenyl-N-1-(2-phenylethyl)piperidin-4-ylacetamide, commonly referred to as methoxyacetylfentanyl. The report aligns with Article 5 of Council Decision 2005/387/JHA, focusing on information exchange, risk assessment, and control of new psychoactive substances. Publications Office. doi:10.2810/786704. ISBN 9789294972569.
- Mounteney J, Giraudon I, Denissov G, Griffiths P (July 2015). “The Emergence of Fentanyls: A Growing Concern in Europe.” Within the pages of The International Journal on Drug Policy, this article explores the increasing prevalence of highly potent fentanyls in Europe, raising critical questions about their impact. Volume 26, Issue 7, presents insights into this troubling trend. doi:10.1016/j.drugpo.2015.04.003. PMID 25976511.
- Armenian P, Vo KT, Barr-Walker J, Lynch KL (October 2017). “Unveiling Fentanyl and Its Analogues: An Extensive Overview.” A comprehensive review published in Neuropharmacology discusses fentanyl, its analogues, and novel synthetic opioids, providing an in-depth analysis of these substances. The paper, found in Neuropharmacology, Volume 134, Part A, delves into the subject extensively. doi:10.1016/j.neuropharm.2017.10.016. PMID 29042317. S2CID 21404877.
- Drug Enforcement Administration, Department of Justice (October 2017). “Restricting Substances: Temporary Classification of ortho-Fluorofentanyl, Tetrahydrofuranyl Fentanyl, and Methoxyacetyl Fentanyl as Schedule I Drugs.” In a temporary amendment and scheduling order detailed in the Federal Register, the Drug Enforcement Administration (DEA) addresses the temporary placement of ortho-Fluorofentanyl, Tetrahydrofuranyl Fentanyl, and Methoxyacetyl Fentanyl into Schedule I of controlled substances. Published in Federal Register, Volume 82, Issue 206. PMID 29091366.