Sumamry
Methylmethaqualone (MMQ) is a compound classified under the quinazolinone group and serves as an analog of methaqualone. It shares similar sedative and hypnotic properties with its parent compound, primarily due to its agonistic influence on the β subtype of the GABAA receptor. Notably, in animal models, Methylmethaqualone has been shown to be approximately three times as potent as its predecessor.
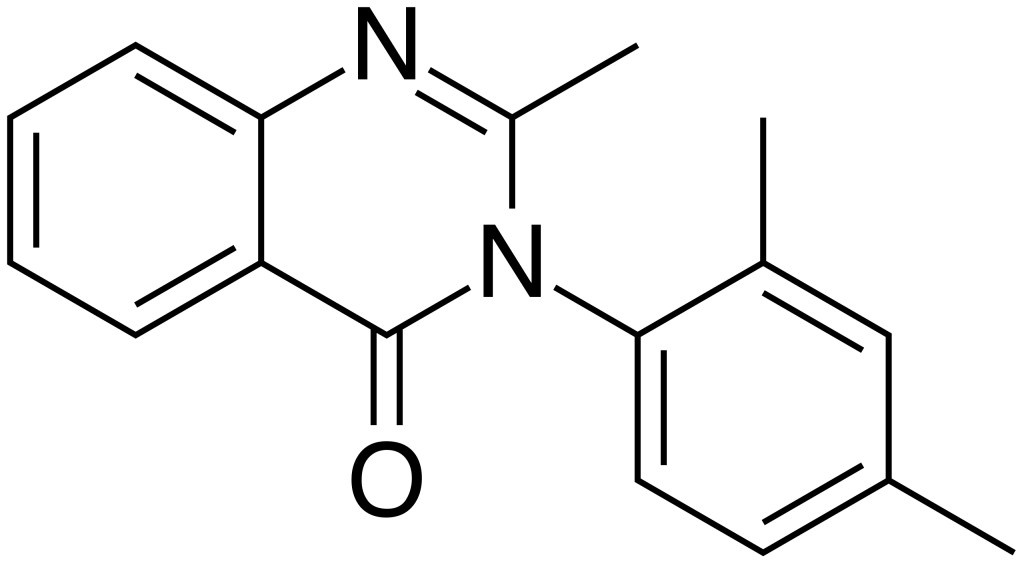
The critical difference between Methylmethaqualone and methaqualone lies in the 4-methylation present on the phenyl ring. In 1999, it was made illegal in Germany, and concurrently, the DEA listed it as a “drug of forensic interest.” However, detailed information about this compound remains limited. It appears that Methylmethaqualone was traded on the black market in Germany, positioning itself as a designer drug analog of methaqualone.
Animal studies have unveiled that Methylmethaqualone can induce convulsions at doses slightly higher than the effective sedative dose. Furthermore, anecdotal reports from human users have affirmed its potential pro-convulsive effects, thereby highlighting the potential hazards associated with excessive intake of this compound. Caution is advised when dealing with substances of this nature due to their potential for adverse effects.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 3244-75-5 |
|---|---|
| PubChem CID | 63382 |
| ChemSpider | 57045 |
| UNII | OBQ1BQR74T |
| CompTox Dashboard (EPA) | DTXSID00172675 |
| Chemical and physical data | |
| Formula | C17H16N2O |
| Molar mass | 264.322 g·mol−1 |
FAQ
1. What is Methylmethaqualone (MMQ)?
Methylmethaqualone is a chemical compound classified under the quinazolinone group and serves as an analog of methaqualone. It shares similar sedative and hypnotic properties with its parent compound.
2. How does Methylmethaqualone compare to Methaqualone in terms of potency?
Methylmethaqualone is approximately three times as potent as methaqualone, as demonstrated in animal models. This increased potency sets it apart from its predecessor.
3. What distinguishes Methylmethaqualone from Methaqualone in terms of chemical structure?
The key structural difference between Methylmethaqualone and methaqualone is the presence of a 4-methylation on the phenyl ring.
4. Is Methylmethaqualone legal in any country?
Methylmethaqualone was made illegal in Germany in 1999. Legal statuses may vary in different regions, so it’s essential to research and understand the specific regulations and laws regarding its use and possession in your country.
5. Why is Methylmethaqualone considered a “drug of forensic interest” by the DEA?
The DEA’s classification of Methylmethaqualone as a “drug of forensic interest” suggests that it may have attracted attention due to potential misuse or its presence in forensic investigations. This categorization implies a degree of concern regarding its use and distribution.
6. How was Methylmethaqualone used and marketed in Germany?
It appears that Methylmethaqualone was sold on the black market in Germany as a designer drug analog of methaqualone, although detailed information about its use and distribution remains limited.
7. What are the potential risks associated with Methylmethaqualone use?
Animal studies have shown that Methylmethaqualone can induce convulsions at doses slightly higher than the effective sedative dose. Anecdotal reports from human users have confirmed its pro-convulsive effects, highlighting the potential hazards associated with excessive intake of this compound. Caution is strongly advised due to these risks.
8. Can Methylmethaqualone be legally prescribed or used for medical purposes?
Methylmethaqualone is not recognized for any approved medical or therapeutic uses and is typically not legally prescribed by healthcare professionals.
9. How can I find more information about Methylmethaqualone and its effects?
To learn more about Methylmethaqualone and its effects, you should rely on reputable sources of scientific literature, authoritative medical archives, and official drug enforcement agencies. Additionally, consult with healthcare professionals for further guidance and information on this substance.
References
- DE Patent 1124504B details a “Verfahren zur Herstellung von 2-Methyl-3-(2′,4′-dimethylphenyl)-4-oxo-3,4-dihydrochinazolin” (Method for the Production of 2-Methyl-3-(2′,4′-Dimethylphenyl)-4-Oxo-3,4-Dihydroquinazoline).
- In a comprehensive review spanning 1992 to 2001, Klein RF and Hays PA explored the “Detection and Analysis of Drugs of Forensic Interest.” This literature review, available in PDF format, was published in the Microgram Journal by the DEA. It can be found in Volume 1, Issues 1–2, on page 60. An archived version of the review is available until July 19, 2011.
- In March 1993, Angelos SA, Lankin DC, Meyers JA, and Raney JK conducted structural identification of a methyl analog of methaqualone using 2-dimensional NMR techniques. Their findings were published in the Journal of Forensic Sciences, Volume 38, Issue 2, on pages 455–465, with a reference DOI: 10.1520/JFS13428J and PMID: 8455002.
- In August 1963, Boltze KH, Dell HD, Lehwald H, Lorenz D, and Rueberg-Schweer M explored “[Substituted 4-Quinazolinone Derivatives As Hypnotics and Anticonvulsants]” in the Arzneimittel-Forschung (in German). This research is available in Volume 13, spanning pages 688–701, with reference to PMID: 14085923.