Summary
MT-45 (also known as IC-6) is an opioid analgesic medication that was initially created in the 1970s by Dainippon Pharmaceutical Co. Its chemical structure is that of a 1-substituted-4-(1,2-phenylmethyl) piperazine derivative, setting it apart from the majority of other opioid drugs. In its racemic form, MT-45 possesses approximately 80% of the potency of morphine, with the primary opioid effects residing in the (S) enantiomer. Notably, this enantiomer’s stereochemistry differs from the related drug lefetamine.
MT-45 has served as a foundational compound for the development of a diverse family of potent opioid drugs. This includes full agonists, partial agonists, and antagonists targeting the three primary opioid receptor subtypes.
Derivatives of MT-45 that have undergone fluorination, such as 2F-MT-45, exhibit significantly greater potency as μ-opioid receptor agonists. Additionally, one of its principal metabolites, 1,2-diphenylethylpiperazine, possesses the ability to block NMDA receptors, further emphasizing the pharmacological versatility of this compound.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 52694-55-0 52694-52-7 (S) enantiomer |
|---|---|
| PubChem CID | 431865 |
| ChemSpider | 381935 |
| UNII | Q2640DPW33 |
| CompTox Dashboard (EPA) | DTXSID60961800 |
| Chemical and physical data | |
| Formula | C24H32N2 |
| Molar mass | 348.534 g·mol−1 |
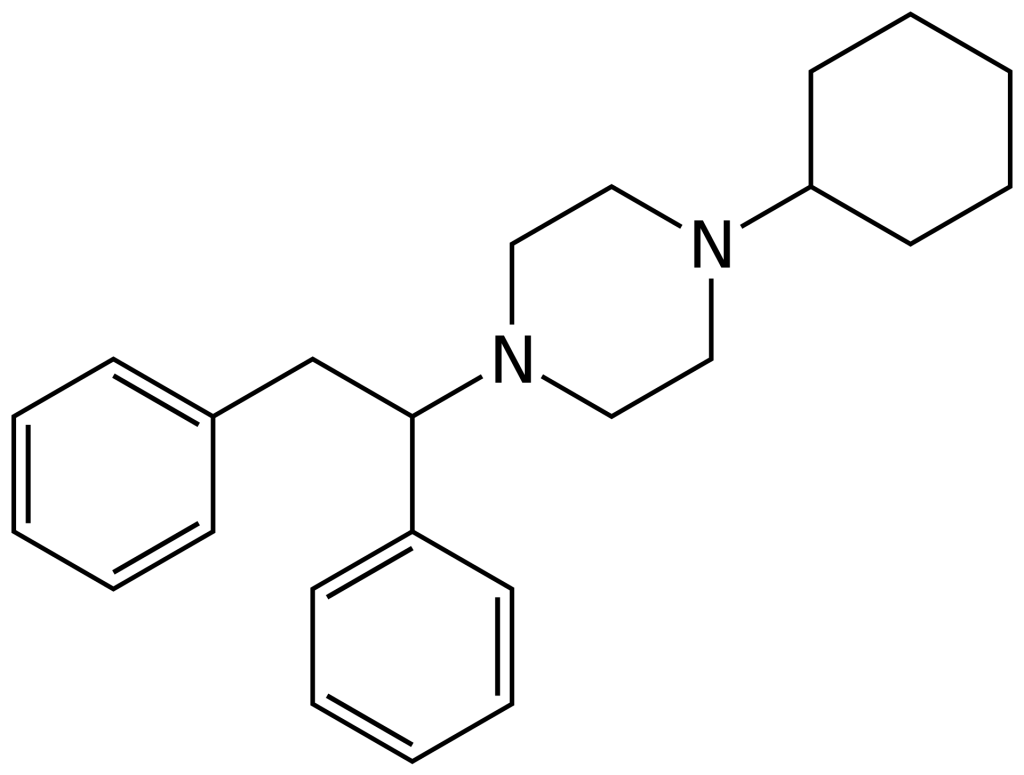
Side effects
The recreational use of MT-45 has been linked to instances of unconsciousness, overdose, and an array of atypical side effects not typically associated with other opioid agonists. These unusual effects encompass conditions like hearing loss, hair depigmentation, alopecia, cataracts, and various skin and nail reactions, including dermatitis and Mees lines. While the exact cause of these side effects remains unclear, there is recognition of a structural resemblance to the withdrawn drug trimaran, which also induced similar adverse reactions.
Legality
- In the United Kingdom, MT-45 was categorized as a class A drug on March 11, 2015.
- The Czech Republic has banned the use of MT-45.
- In Canada, the Controlled Drugs and Substances Act was modified in 2016 to classify MT-45 as a Schedule I substance. Possession without legal authorization can lead to a maximum prison term of 7 years. Health Canada also updated the Food and Drug Regulations in May 2016 to designate MT-45 as a restricted drug. In Canada, only law enforcement agencies, individuals with exemption permits, or authorized institutions may possess this substance.
- In the United States, the Drug Enforcement Administration (DEA) placed MT-45 in Schedule I of the Controlled Substances Act, which became effective on January 12, 2018.
FAQ
1. What is MT-45?
- MT-45 is an opioid analgesic medication originally developed by Dainippon Pharmaceutical Co. in the 1970s. It possesses distinct chemical properties compared to many other opioid drugs.
2. What are the effects of MT-45?
- MT-45 can produce typical opioid effects, such as pain relief and feelings of euphoria and sedation.
3. Are there unusual side effects associated with MT-45 use?
- Yes, MT-45 use has been linked to a range of atypical side effects not commonly seen with other opioids. These include hearing loss, hair depigmentation, alopecia, cataracts, and various skin and nail reactions like dermatitis and Mees lines.
4. Why do these unusual side effects occur with MT-45 use?
- The exact cause of these unusual side effects is not fully understood. However, it’s been noted that MT-45 shares a structural similarity with a withdrawn drug called trimaran, which also causes similar side effects.
5. Is MT-45 legal for recreational use?
- The legal status of MT-45 varies by country. In many regions, it has been classified as a controlled or banned substance due to its potential for abuse and adverse effects.
6. Is MT-45 available for medical use?
- MT-45 is not approved for medical use in most countries due to its unusual side effects and potential health risks.
7. What is the legal status of MT-45 in the United Kingdom?
- In the UK, MT-45 was classified as a class A drug on March 11, 2015.
8. Is MT-45 banned in the Czech Republic?
- Yes, the use of MT-45 is prohibited in the Czech Republic.
9. How is MT-45 regulated in Canada?
- In Canada, MT-45 is classified as a Schedule I substance under the Controlled Drugs and Substances Act. Possession without legal authority can result in significant penalties, and only specific authorized entities may possess the sense.
10. What is the legal status of MT-45 in the United States?
- In the United States, the Drug Enforcement Administration (DEA) placed MT-45 in Schedule I of the Controlled Substances Act, making it illegal for recreational use. This change became effective on January 12, 2018.
References
- In 1976, a US patent (3957788) was issued for “1-Substituted-4-(1,2-diphenylethyl)piperazine derivatives and their salts.” The patent was published on January 15, 1975, and officially issued on May 18, 1976.
- Natsuka K, Nakamura H, Uno H, Umemoto S (December 1975) conducted “Studies on 1-substituted 4-(1,2-diphenylethyl)piperazine derivatives and their analgesic activities. 1” as published in the Journal of Medicinal Chemistry, Volume 18, Issue 12, with pages 1240–1244.
- Nakamura H, Shimizu M (May 1976) engaged in a “Comparative study of 1-cyclohexyl-4-(1,2-diphenylethyl)-piperazine and its enantiomorphs on analgesic and other pharmacological activities in experimental animals.” This research was featured in Archives Internationales de Pharmacodynamie et de Thérapie, Volume 221, Issue 1, with pages 105–121.
- Another US Patent (4080453) is associated with this research field.
- Natsuka K, Nakamura H, Negoro T, Uno H, Nishimura H (December 1978) conducted “Studies on 1-substituted 4-(1,2-diphenylethyl)piperazine derivatives and their analgesic activities. 2. Structure-activity relationships of 1-cycloalkyl-4-(1,2-diphenylethyl)piperazines” in the Journal of Medicinal Chemistry, Volume 21, Issue 12, with pages 1265–1269.
- Shimokawa N, Nakamura H, Shimakawa K, Minami H, Nishimura H (January 1979) explored “Studies on analgesic agents. 1.1a Preparation of 1,2-diphenyl-2-(4-substituted 1-piperazinyl)ethanol derivatives and structure-activity relationships” in the Journal of Medicinal Chemistry, Volume 22, Issue 1, with pages 58–63.
- Nakamura H, Ishii D, Yokoyama Y, Motoyoshi S, Natsuka K, Shimizu M (September 1980) delved into “Analgesic and other pharmacological activities of a new narcotic antagonist analgesic (−)-1-(3-methyl-2-butenyl)-4-[2-(3-hydroxyphenyl)-1-phenylethyl]-piperazine and its enantiomorph in experimental animals.” This research was published in The Journal of Pharmacy and Pharmacology, Volume 32, Issue 9, with pages 635–642.
- Nozaki M, Niwa M, Imai E, Hori M, Fujimura H (1983) explored “(1,2-Diphenylethyl) piperazines as potent opiate-like analgesics; the unusual relationships between stereoselectivity and affinity to opioid receptor” in Life Sciences, Volume 33, Supplement 1, with pages 431–434.
- Natsuka K, Nakamura H, Nishikawa Y, Negoro T, Uno H, Nishimura H (October 1987) conducted “Synthesis and structure-activity relationships of 1-substituted 4-(1,2-diphenylethyl)piperazine derivatives having narcotic agonist and antagonist activity” in the Journal of Medicinal Chemistry, Volume 30, Issue 10, with pages 1779–1787.
- Natsuka K, Nishikawa Y, Nakamura H (December 1999) examined the “Roles of two basic nitrogen atoms in 1-substituted 4-(1,2-diphenylethyl)piperazine derivatives in production of opioid agonist and antagonist activities” in Chemical & Pharmaceutical Bulletin, Volume 47, Issue 12, with pages 1790–1793.
- Baptista-Hon DT, Smith M, Singleton S, Antonides LH, Nic Daeid N, McKenzie C, Hales TG (August 2020) conducted research on the “Activation of μ-opioid receptors by MT-45 (1-cyclohexyl-4-(1,2-diphenylethyl)piperazine) and its fluorinated derivatives” in the British Journal of Pharmacology, Volume 177, Issue 15, with pages 3436–3448.
- Helander A, Bäckberg M, Beck O (2014) reported on “MT-45, a new psychoactive substance associated with hearing loss and unconsciousness” in Clinical Toxicology, Volume 52, Issue 8, with pages 901–904.
- Helander A, Bradley M, Hasselblad A, Norlén L, Vassilaki I, Bäckberg M, Lapins J (April 2017) documented “Acute skin and hair symptoms followed by severe, delayed eye complications in subjects using the synthetic opioid MT-45” in The British Journal of Dermatology, Volume 176, Issue 4, with pages 1021–1027.
- Wallach JV, Morris H, Brandt SD (August 2017) explored “Is nitrogen mustard contamination responsible for the reported MT-45 toxicity?” in The British Journal of Dermatology, Volume 177, Issue 2, with pages 594–595.
- Helander A, Bradley M, Lapins J (August 2017) provided a reply on the topic of “Is nitrogen mustard contamination responsible for the reported MT-45 toxicity?” in The British Journal of Dermatology, Volume 177, Issue 2, with page 595.
- Solimini R, Pichini S, Pacifici R, Busardò FP, Giorgetti R (2018) delved into the “Pharmacotoxicology of Non-fentanyl Derived New Synthetic Opioids” in Frontiers in Pharmacology, Volume 9, with article number 654.
- McKenzie C, Sutcliffe OB, Read KD, Scullion P, Epemolu O, Fletcher D, et al. (2018) conducted research on the “Chemical synthesis, characterisation and in vitro and in vivo metabolism of the synthetic opioid MT-45 and its newly identified fluorinated analogue 2F-MT-45 with metabolite confirmation in urine samples from known drug users” in Forensic Toxicology, Volume 36, Issue 2, with pages 359–374.
- “Circular 003/2015: a change to the Misuse of Drugs Act 1971: control of MT-45 and 4,4′-DMAR” was released by the UK Home Office on February 20, 2015.
- “Látky, o které byl doplněn seznam č. 4 psychotropních látek (příloha č. 4 k nařízení vlády č. 463/2013 Sb.)” provides information on substances, including MT-45, added to the list of psychotropic substances in the Czech Republic.
- “Regulations Amending the Food and Drug Regulations (Parts G and J — Lefetamine, AH-7921, MT-45 and W-18)” outlines amendments to the Food and Drug Regulations in Canada.
- “2017 – Final Order: Placement of MT-45 into Schedule I” provides details about the placement of MT-45 into Schedule I in the United States.