Summary
N-Desalkylflurazepam, also referred to as norflurazepam, is classified as a benzodiazepine analog and serves as an active metabolite for several other benzodiazepine drugs, which encompass flurazepam, flutoprazepam, fludiazepam, midazolam, flutazolam, quazepam, and ethyl loflazepate.
This compound is known for its long-lasting effects and tends to accumulate in the body. It binds to a range of benzodiazepine receptor subtypes without displaying selective preferences.
Starting in 2016, N-Desalkylflurazepam has been marketed and distributed as a designer drug.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 2886-65-9 |
|---|---|
| PubChem CID | 4540 |
| ChemSpider | 4381 |
| UNII | X9U41NXR6M |
| ChEMBL | ChEMBL974 |
| CompTox Dashboard (EPA) | DTXSID30183057 |
| ECHA InfoCard | 100.018.863 |
| Chemical and physical data | |
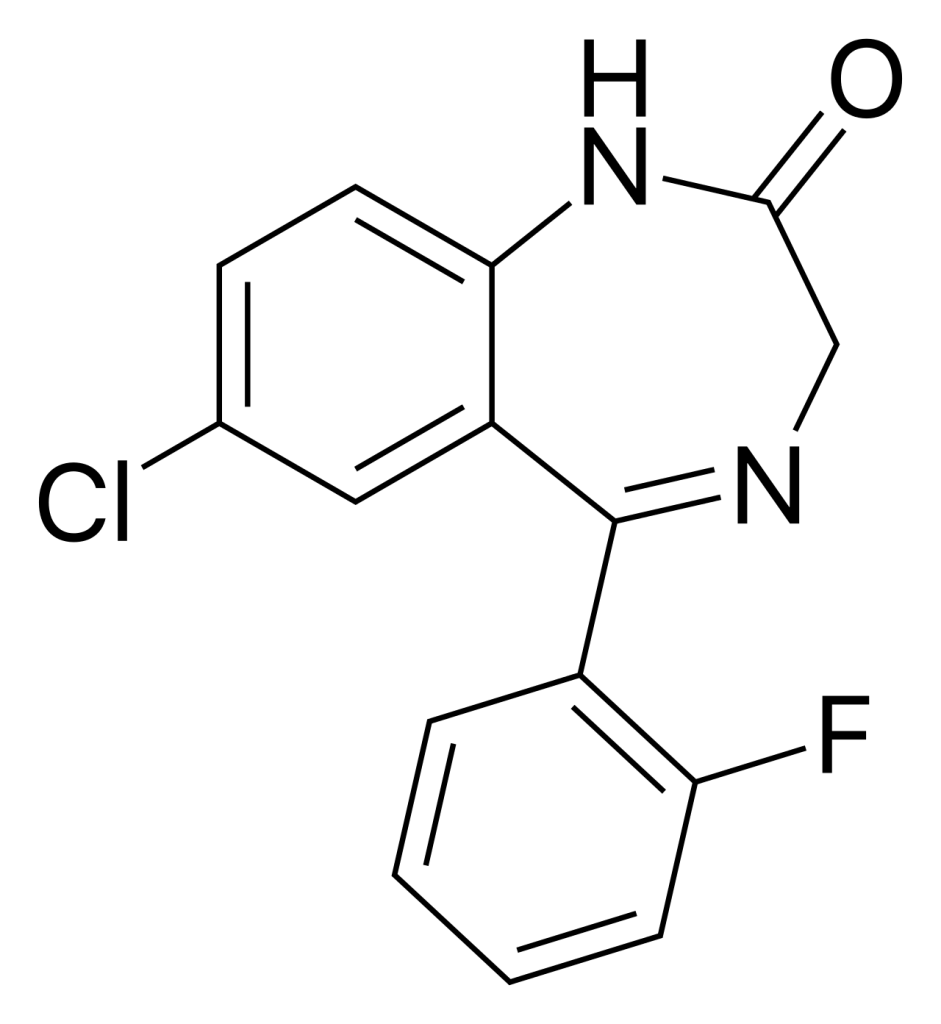
| Formula | C15H10ClFN2O |
| Molar mass | 288.71 g·mol−1 |
FAQ
1. What is N-Desalkylflurazepam?
N-desalkylflurazepam, also known as norflurazepam, is a chemical compound classified as a benzodiazepine analogue. It is an active metabolite of various other benzodiazepine drugs.
2. How does N-Desalkylflurazepam differ from other benzodiazepines?
N-desalkylflurazepam is distinct in that it serves as an active metabolite for several benzodiazepine drugs, meaning it is produced as a result of the body processing these other substances.
3. Which benzodiazepine drugs are metabolized into N-Desalkylflurazepam?
N-Desalkylflurazepam is an active metabolite of several benzodiazepine drugs, including flurazepam, flutoprazepam, fludiazepam, midazolam, flutazolam, quazepam, and ethyl loflazepate.
4. What are the effects of N-desalkylflurazepam?
As a benzodiazepine analogue, N-Desalkylflurazepam exhibits sedative and anxiolytic (anxiety-reducing) effects. It can induce relaxation and alleviate symptoms of anxiety.
5. Is N-desalkylflurazepam available as a prescription medication?
N-desalkylflurazepam is typically not prescribed as a standalone medication. It is primarily an active metabolite and is not commonly used for medical purposes.
6. How long do the effects of N-desalkylflurazepam last?
N-desalkylflurazepam is known for its long-lasting effects. The duration of its effects can vary from person to person but is generally longer compared to some other benzodiazepines.
7. Does N-desalkylflurazepam have the potential for abuse or addiction?
Like other benzodiazepines, N-desalkylflurazepam has the potential for abuse and can lead to physical and psychological dependence if misused. It is essential to use this substance only under medical supervision.
8. Can N-desalkylflurazepam be detected in drug tests?
N-desalkylflurazepam can be detected in drug tests, especially those designed to identify benzodiazepines. It is essential to be aware of its potential presence in drug screening.
9. Is N-desalkylflurazepam available as a designer drug?
Yes, N-Desalkylflurazepam has been marketed and sold as a designer drug since 2016. It is essential to exercise caution when considering its use due to the associated legal and health risks.
10. Where can I find more information about N-desalkylflurazepam?
For more information about N-Desalkylflurazepam, consult scientific literature, medical professionals, or drug education resources. Always prioritize your health and safety when seeking information about psychoactive substances.
References
- SciFinder database record for CAS#2886-65-9.
- Riva R, de Anna M, Albani F, Baruzzi A (March 1981). “Swift Quantification of Flurazepam and Its Principal Metabolite, N-Desalkylflurazepam, in Human Plasma Using Gas-Liquid Chromatography with Electron-Capture Detection.” Published in the Journal of Chromatography. Volume 222, Issue 3, Pages 491–5. doi: 10.1016/S0378-4347(00)84153-5. PMID 7228960.
- Barzaghi N, Leone L, Monteleone M, Tomasini G, Perucca E (1989). “Pharmacokinetics of Flutoprazepam, a Novel Benzodiazepine Drug, in Healthy Individuals.” Published in the European Journal of Drug Metabolism and Pharmacokinetics. Volume 14, Issue 4, Pages 293–8. doi: 10.1007/bf03190114. PMID 2633923. S2CID 20710732.
- Descotes J, editor (December 1996). “Human Toxicology” (1st ed.). Published by Elsevier Science. Page 43.
- Vogt S, Kempf J, Buttler J, Auwärter V, Weinmann W (2013). “Detection of Desalkylflurazepam in Patients’ Samples Following High-Dose Midazolam Treatment.” Published in Drug Testing and Analysis. Volume 5, Issues 9–10, Pages 745–7. doi: 10.1002/dta.1484. PMID 23713025.
- Miyaguchi H, Kuwayama K, Tsujikawa K, Kanamori T, Iwata YT, Inoue H, Kishi T (February 2006). “A Method for Screening Various Sedative-Hypnotics in Serum Using Liquid Chromatography/Single Quadrupole Mass Spectrometry.” Published in Forensic Science International. Volume 157, Issue 1, Pages 57–70. doi: 10.1006/j.forsciint.2005.03.011. PMID 15869852.
- Nikaido AM, Ellinwood EH (1987). “Comparative Effects of Quazepam and Triazolam on Cognitive-Neuromotor Performance.” Published in Psychopharmacology. Volume 92, Issue 4, Pages 459–64. doi: 10.1007/bf00176478. PMID 2888152. S2CID 13162524.
- Ba BB, Iliadis A, Cano JP (1989). “Pharmacokinetic Modeling of Ethyl Loflazepate (Victan) and Its Principal Active Metabolites.” Published in the Annals of Biomedical Engineering. Volume 17, Issue 6, Pages 633–46. doi: 10.1007/bf02367467. PMID 2574017. S2CID 31310535.
- Davi H, Guyonnet J, Necciari J, Cautreels W (July 1985). “Quantification of Circulating Ethyl Loflazepate Metabolites in Baboons Using Radio-High-Performance Liquid Chromatography with Injection of Crude Plasma Samples: A Comparison with Solvent Extraction and Thin-Layer Chromatography.” Published in the Journal of Chromatography. Volume 342, Issue 1, Pages 159–65. doi: 10.1006/j.forsciint.2005.03.011. PMID 2864352.
- Manchester KR, Maskell PD, and Waters L (March 2018). “Experimental vs. Theoretical Log D7.4, pKa, and Plasma Protein Binding Values for Benzodiazepines Appearing as New Psychoactive Substances.” Published in Drug Testing and Analysis. Volume 10, Issue 8, Pages 1258–1269. doi: 10.1002/dta.2387. PMID 29582576.