Summary
Nabilone, marketed under the trade name Cesamet and other brands, is a synthetic cannabinoid recognized for its therapeutic applications as an antiemetic and as a supplementary analgesic in managing neuropathic pain. This compound emulates tetrahydrocannabinol (THC), the primary psychoactive component naturally found in the Cannabis plant.
In the United States, the Food and Drug Administration has approved nabilone specifically for countering chemotherapy-induced nausea and vomiting. In other regions, such as Canada, it is extensively employed as an additional treatment for chronic pain control. A wealth of clinical trials and case studies has shown its moderate efficacy in alleviating conditions like fibromyalgia and multiple sclerosis.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 51022-71-0 |
|---|---|
| PubChem CID | 5284592 |
| DrugBank | DB00486 |
| ChemSpider | 4447641 |
| UNII | 2N4O9L084N |
| KEGG | D05099 |
| ChEMBL | ChEMBL947 |
| ECHA InfoCard | 100.164.824 |
| Chemical and physical data | |
| Formula | C24H36O3 |
| Molar mass | 372.549 g·mol−1 |
Medical uses
Nabilone is employed in the management of nausea and vomiting in individuals undergoing chemotherapy. It has also demonstrated moderate effectiveness in alleviating symptoms of fibromyalgia, as supported by a 2011 systematic review on cannabinoids for chronic pain that indicated evidence of safety and modest efficacy for specific conditions.
Clinical trials with nabilone have primarily focused on various medical settings, including movement disorders like parkinsonism, chronic pain, dystonia, spasticity, neurological disorders, multiple sclerosis, and chemotherapy-induced nausea. Moreover, it has proven to be an effective treatment for inflammatory bowel disease, notably ulcerative colitis.
In a particular study involving regular cannabis users, oral nabilone at doses of 4, 6, and 8 mg produced sustained mood elevation and psychomotor slowing in a dose-dependent manner, comparable to the effects of 10 or 20 mg of oral dronabinol (THC). Nabilone exhibited a slower onset of peak action and a more robust dose-dependent response, which was attributed to its enhanced bioavailability.
A study comparing nabilone with metoclopramide, conducted before the advent of modern 5-HT3 antagonist antiemetic drugs like ondansetron, revealed that patients receiving cisplatin chemotherapy preferred metoclopramide. In contrast, those undergoing carboplatin chemotherapy favored nabilone for the control of nausea and vomiting.
Nabilone has also been explored for its potential to alleviate nightmares associated with post-traumatic stress disorder. However, studies beyond nine weeks are lacking, and the effects of long-term use remain uncertain. Additionally, nabilone has been utilized in the treatment of medication overuse headaches.
Side effects
Nabilone may, counterintuitively, exacerbate postoperative pain rather than alleviate it. In the context of fibromyalgia treatment, adverse effects impose constraints on the dosage that can be safely administered. These adverse effects encompass but are not restricted to, sensations of dizziness or vertigo, euphoria, drowsiness, dry mouth, ataxia, disruptions in sleep patterns, headaches, nausea, disorientation, depersonalization, hallucinations, and asthenia.
Pharmacology
- Pharmacodynamics:
- Nabilone operates as a partial agonist on the cannabinoid CB1 and CB2 receptors.
- Pharmacokinetics:
- Nabilone is typically administered in doses of 1 or 2 mg multiple times per day, with a cumulative daily limit of 6 mg. It exhibits complete absorption following oral ingestion and has a strong affinity for plasma proteins. Multiple cytochrome P450 enzymes play a significant role in extensively metabolizing nabilone into a variety of metabolites, some of which remain incompletely characterized.
- Chemistry:
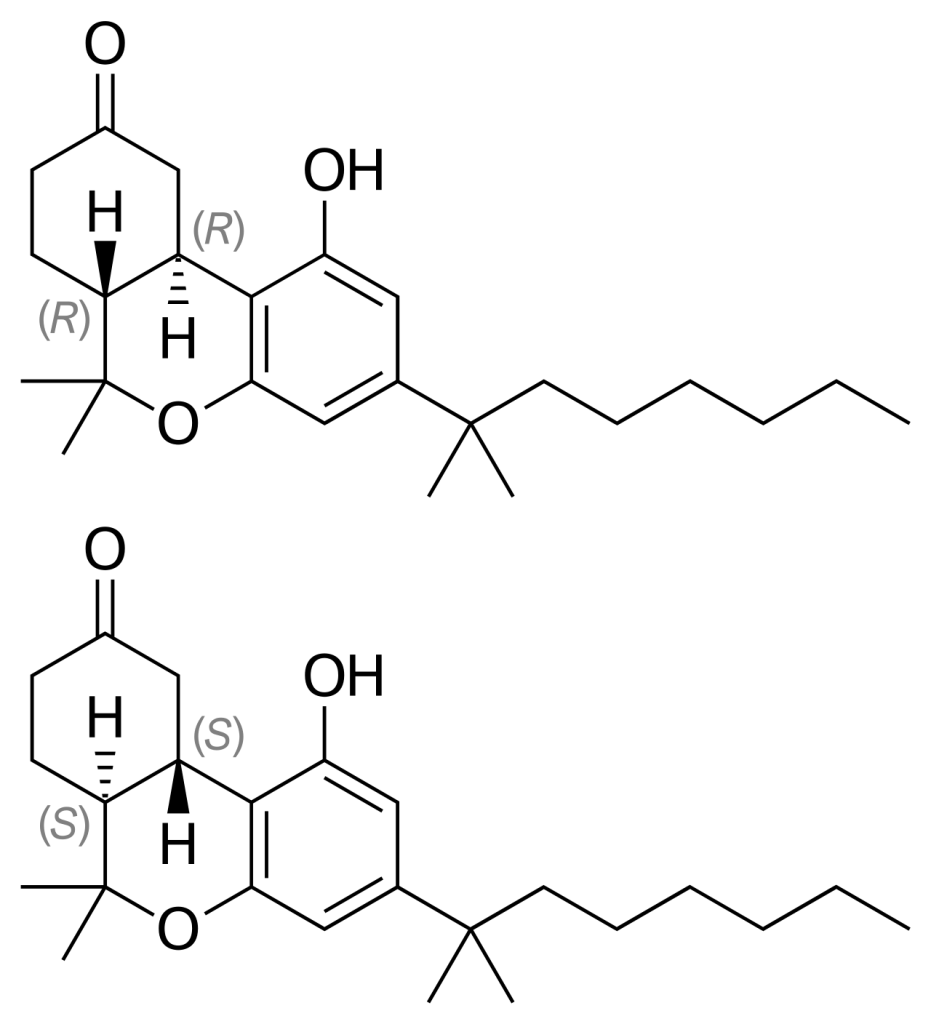
- Nabilone is composed of a racemic mixture that includes both (S, S)-(+)- and (R, R)-(−)-isomers.
History
Nabilone’s journey began with its development by Eli Lilly and Company, leading to its initial approval by Health Canada in 1981. Shortly after that, it gained approval in Mexico, the United Kingdom, and Germany. Although it obtained FDA approval for marketing in 1985, this approval was withdrawn in 1989 due to commercial considerations. Subsequently, Valeant Pharmaceuticals acquired the rights from Lilly in 2004. Valeant’s attempts to secure FDA approval in 2005 were unsuccessful, but they ultimately succeeded in 2006.
In 2007, Valeant extended its reach by acquiring the rights to market nabilone in the United Kingdom and the European Union from Cambridge Laboratories.
In 2013, nabilone received approval in Austria for its use in managing chemotherapy-induced nausea. Prior to this, it had already obtained approval in Spain for the same indication. It was authorized in Belgium for treating glaucoma, spasticity associated with multiple sclerosis, AIDS-related wasting, and chronic pain.
FAQ
1. What is Nabilone?
- Nabilone is a synthetic cannabinoid medication that is used for various therapeutic purposes, including managing nausea and vomiting, especially in chemotherapy patients, and as an adjunct in pain management.
2. How does Nabilone work?
- Nabilone works by interacting with the body’s endocannabinoid system. It acts as a partial agonist on the cannabinoid CB1 and CB2 receptors, which are involved in regulating various physiological processes.
3. What conditions are Nabilone used to treat?
- Nabilone is primarily used to control nausea and vomiting in individuals undergoing chemotherapy. It has also shown effectiveness in managing symptoms of fibromyalgia and has been explored for conditions like multiple sclerosis, inflammatory bowel disease, and post-traumatic stress disorder.
4. Are there any side effects associated with Nabilone?
- Yes, Nabilone can cause side effects. Common side effects include dizziness, euphoria, drowsiness, dry mouth, ataxia (lack of coordination), sleep disturbances, headaches, nausea, disorientation, depersonalization, hallucinations, and asthenia (lack of strength). It is essential to discuss potential side effects with your healthcare provider.
5. How is Nabilone administered?
- Nabilone is typically administered orally in doses of 1 or 2 mg multiple times a day, with a maximum daily dose of 6 mg. Your healthcare provider will determine the appropriate dosage based on your medical condition and individual needs.
6. Can Nabilone be used for a prolonged period?
- Long-term use of Nabilone beyond nine weeks has not been extensively studied for specific conditions. If you are prescribed Nabilone for an extended period, your healthcare provider will monitor its effects and any potential side effects closely.
7. Who should not take Nabilone?
- Nabilone may not be suitable for individuals with a history of substance abuse, as it can have psychoactive effects. It should also be used with caution in individuals with certain mental health conditions. Always consult with your healthcare provider to determine if Nabilone is safe and appropriate for you.
8. Is Nabilone legal and available everywhere?
- The legal status of Nabilone varies by country and region. It may be approved for specific medical indications in some places and not in others. Please consult with your healthcare provider or local regulatory authorities to understand its availability and legal status in your area.
9. Is Nabilone the same as medical marijuana (cannabis)?
- No, Nabilone is a synthetic cannabinoid medication, whereas medical marijuana uses natural compounds from the cannabis plant. While both interact with the endocannabinoid system, they have different chemical compositions and are used for different purposes.
10. Can Nabilone be used for recreational purposes?
- Nabilone is a prescription medication intended for medical use. It should only be used under the guidance of a healthcare provider to treat specific medical conditions and is not meant for recreational use. Misuse or unauthorized use can lead to health risks and legal consequences.
References
- “Nabilone – Insights from Adis”
- “Patient Guidance on Nabilone: What You Need to Know”
- “Official Nabilone Label – FDA Document from May 2006”
- “Exploring the Endocannabinoid System, Cannabinoids, and Their Role in Pain Management” – An In-Depth Review
- “Reducing Spasticity-Related Pain: Nabilone’s Efficacy in a Double-Blind Placebo-Controlled Trial”
- “Cannabis and Cannabinoids in Managing Multiple Sclerosis Symptoms: A Systematic Review of Reviews”
- “Nabilone’s Summary of Product Characteristics: A Detailed Look” (eMC)
- “Cannabinoids for Chronic Non-Cancer Pain: Insights from a Comprehensive Review of Randomized Trials”
- “Subjective, Cognitive, and Cardiovascular Effects of Nabilone and Dronabinol in Cannabis Users”
- “Comparing Nabilone and Metoclopramide: A Study on Controlling Chemotherapy-Induced Nausea and Vomiting”
- “Cannabinoids for Nausea and Vomiting in Cancer Patients: A Comprehensive Cochrane Review”
- “Long-term Nabilone Use: Clinical Effectiveness and Safety – A Thorough Review”
- “Nabilone’s Role in Medication Overuse Headache: Preliminary Results from a Double-Blind Trial”
- “Therapeutic Potential and Safety Considerations: Clinical Use of Synthetic Cannabinoids”
- “Impact of Partial CB1 Receptor Agonists on Cognition: A Meta-Analysis of Human Studies”
- “Medical Cannabis Use for HIV/AIDS Patients: Reducing Morbidity and Mortality – A Cochrane Review”
- “Health Canada’s Insights on Nabilone – Updated Information as of July 2023”
- “Valeant’s Return of Synthetic Cannabinoid to the USA – Pharma Industry News”
- “FDA’s Decision on Valeant’s Anti-Nausea Drug – A Critical Milestone”
- “Cambridge Labs’ Transfer of Nabilone to Valeant – A Strategic Industry Move”
- “Cannabis Laws and Scheduling in Europe – An Overview of the MedicalMarijuana.eu Report”