Summaru
Pagoclone, a member of the cyclopyrrolone family, is classified as an anxiolytic agent, sharing its lineage with more familiar drugs like the sleep aid zopiclone. This compound was initially synthesized by a team of French researchers working for Rhone-Poulenc & Rorer S.A. It falls within the nonbenzodiazepine category, which, despite yielding effects akin to traditional benzodiazepines, features distinct chemical structures. Notably, pagoclone has yet to reach the commercial market.
In terms of its pharmacological action, pagoclone exhibits a robust affinity (ranging from 0.7 to 9.1 nM) for the benzodiazepine binding site within human GABAA receptors, involving α1, α2, α3, or α5 subunits. It acts as a partial agonist at receptors containing α1, α2, and α5 subunits while functioning as a full agonist at those featuring the α3 subunit. In laboratory tests with rats, a significant metabolite known as 5′-hydroxypagoclone was identified. Interestingly, this metabolite displayed greater efficacy at the α1 subtype compared to the parent compound, exhibiting substantial anxiolytic-like effects and inducing sedation. Unlike zopiclone, pagoclone exerts anxiolytic effects with minimal sedative or amnestic consequences at lower doses, typically ranging from 0.3mg to 1.2mg per day.
Renowned pharmacologist David Nutt has proposed pagoclone as a potential basis for developing a more socially amicable drug. It is known to elicit the positive aspects of alcohol consumption, such as relaxation and sociability while sidestepping the negative consequences like aggression, memory loss, nausea, impaired coordination, and liver damage. Moreover, the effects of pagoclone can be rapidly reversed through the administration of flumazenil, a known antidote for benzodiazepine overdose. Nutt’s studies, which Indevus has funded, emphasize the promising potential of pagoclone, thereby generating interest in its production. Nevertheless, the long-term safety of pagoclone remains unassessed, and concerns surrounding its abuse potential persist. It is estimated that pagoclone carries a level of abuse potential similar to, or slightly lower than, diazepam, primarily due to its relatively weaker sedative properties. Despite these merits, commercial development of pagoclone remains improbable due to lingering apprehensions about its potential for abuse.
Worth noting is that pagoclone was initially explored as a treatment for enhancing speech fluency in individuals with stammers. However, this avenue of research was ultimately abandoned after unsatisfactory outcomes in Phase II clinical trials.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 133737-32-3 |
|---|---|
| PubChem CID | 131664 |
| ChemSpider | 116335 |
| UNII | 38VAG2SA33 |
| KEGG | D02616 |
| ChEMBL | ChEMBL2104745 |
| Chemical and physical data | |
| Formula | C23H22ClN3O2 |
| Molar mass | 407.90 g·mol−1 |
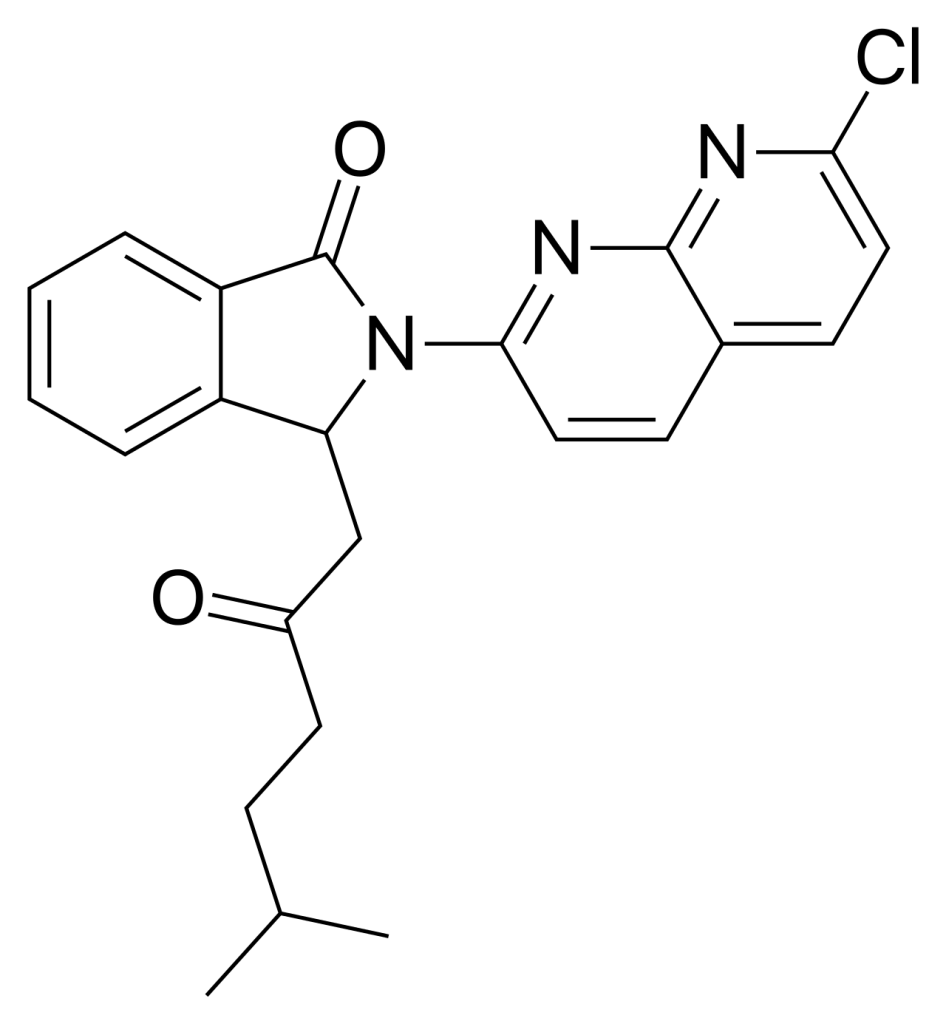
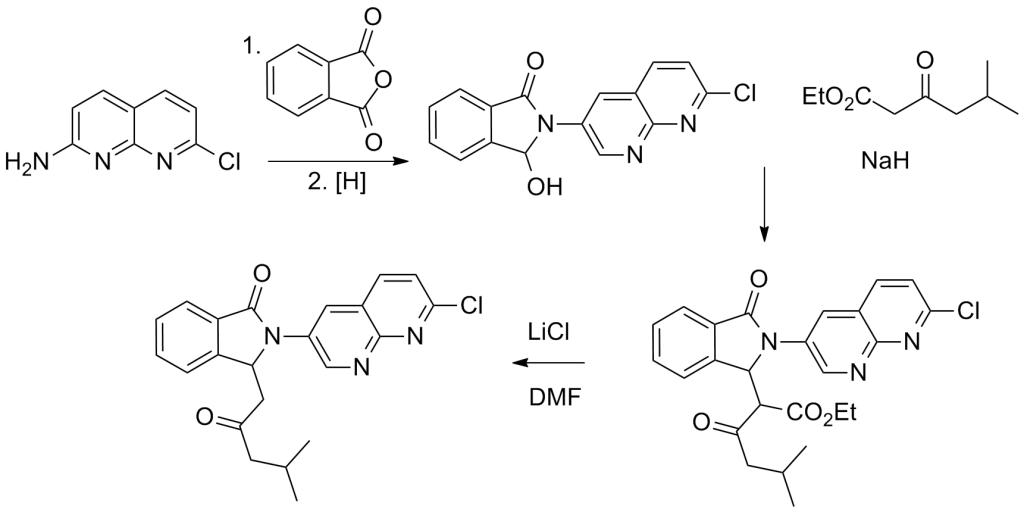
Synthesis
The reaction of 2-Amino-7-chloro-1,8-naphthyridine with phthalic anhydride results in the formation of the corresponding phthalimide. By selectively reducing one of the imide carbonyl groups, the corresponding alcohol is obtained. Additionally, when reacted with the carbanion derived from Ethyl 5-methyl-3-oxohexanoate, it leads to the product through the displacement of the hydroxyl group. This process may also involve the intermediate acrylate formed from the aldol reaction of the opened-ring imide.
FAQ
- What is Pagoclone?
- Pagoclone is a chemical compound belonging to the cyclopyrrolone family. It is primarily recognized as an anxiolytic agent, used for its potential in managing anxiety.
- How does Pagoclone work?
- Pagoclone affects the gamma-aminobutyric acid (GABA) neurotransmitter system. It binds to specific receptors (GABAA receptors) in the brain, which helps reduce anxiety and induce a calming effect.
- Is Pagoclone available as a medication?
- No, Pagoclone was never commercialized, and it is not available as a prescription medication.
- What are the potential applications of Pagoclone?
- Although Pagoclone was not developed for medical use, it has been explored for its potential in creating socially amicable drugs, offering the relaxing and friendly effects of alcohol without its adverse side effects.
- Are there any safety concerns associated with Pagoclone?
- The long-term safety of Pagoclone has not been thoroughly assessed. Additionally, concerns about its potential for abuse persist.
- What is the abuse potential of Pagoclone?
- It is estimated that Pagoclone carries an abuse potential similar to, or slightly lower than, diazepam, a common benzodiazepine. Its relatively weaker sedative effects contribute to this assessment.
- Who is David Nutt, and what is his connection to Pagoclone?
- David Nutt is a pharmacologist who has suggested Pagoclone as a basis for creating more socially acceptable drugs. He has conducted studies highlighting its potential, and some of these studies were financed by Indevus, a company interested in the compound’s production.
- Was Pagoclone ever used in clinical trials for any medical conditions?
- Pagoclone was trialed for improving speech fluency in individuals with stammers. However, research for this application was discontinued after disappointing results in Phase II clinical trials.
- Is Pagoclone reversible if its effects need to be counteracted?
- Yes, Pagoclone’s effects can be rapidly reversed using flumazenil, which is already employed as an antidote for benzodiazepine overdose.
- Is Pagoclone currently under development for any specific applications or uses?
- As of my knowledge, the cutoff date was in January 2022; there were no known developments or plans for commercializing Pagoclone due to concerns about its abuse potential. It’s essential to verify if there have been any updates on its status since then.
References
- US 5498716, David-Comte MT, Roussel G, “2-Amino naphthyridine derivative, its preparation and its use”, issued 12 March 1996, assigned to Rhone Poulenc Rorer SA.
- Atack JR, Pike A, Marshall G, Stanley J, Lincoln R, Cook SM, et al. (May 2006). “The in vivo properties of pagoclone in rat are most likely mediated by 5′-hydroxy pagoclone”. Neuropharmacology. 50 (6): 677–689. doi:10.1016/j.neuropharm.2005.11.014. PMID 16430927. S2CID 12090552.
- Atack JR (May 2005). “The benzodiazepine binding site of GABA(A) receptors as a target for the development of novel anxiolytics”. Expert Opinion on Investigational Drugs. 14 (5): 601–618. doi:10.1517/13543784.14.5.601. PMID 15926867. S2CID 22793644.
- Atack JR (August 2003). “Anxioselective compounds acting at the GABA(A) receptor benzodiazepine binding site”. Current Drug Targets. CNS and Neurological Disorders. 2 (4): 213–232. doi:10.2174/1568007033482841. PMID 12871032.
- Nutt DJ (May 2006). “Alcohol alternatives–a goal for psychopharmacology?”. Journal of Psychopharmacology. 20 (3): 318–320. doi:10.1177/0269881106063042. PMID 16574703. S2CID 44290147.
- Lingford-Hughes A, Wilson SJ, Feeney A, Grasby PG, Nutt DJ (August 2005). “A proof-of-concept study using [11C]flumazenil PET to demonstrate that pagoclone is a partial agonist”. Psychopharmacology. 180 (4): 789–791. doi:10.1007/s00213-005-0060-1. PMID 15986186. S2CID 35569523.
- de Wit H, Vicini L, Haig GM, Hunt T, Feltner D (June 2006). “Evaluation of the abuse potential of pagoclone, a partial GABAA agonist”. Journal of Clinical Psychopharmacology. 26 (3): 268–73. doi:10.1097/01.jcp.0000218983.61683.96. PMID 16702891. S2CID 33351598.
- Maguire G, Franklin D, Vatakis NG, Morgenshtern E, Denko T, Yaruss JS, et al. (February 2010). “Exploratory randomized clinical study of pagoclone in persistent developmental stuttering: the EXamining Pagoclone for peRsistent dEvelopmental Stuttering Study”. Journal of Clinical Psychopharmacology. 30 (1): 48–56. doi:10.1097/jcp.0b013e3181caebbe. PMID 20075648. S2CID 29633149.