Summary
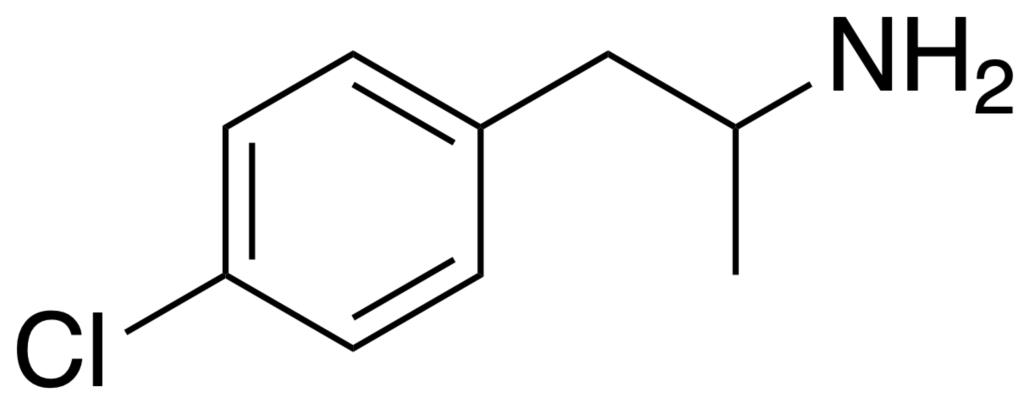
Para-chloroamphetamine (PCA), also recognized as 4-chloroamphetamine (4-CA), falls within the category of substituted amphetamines and serves as a monoamine releaser. It shares similarities with MDMA but is notably more neurotoxic, primarily attributed to the uncontrolled release of both serotonin and dopamine by a metabolite.
This compound is frequently employed by neurobiologists as a neurotoxin for research purposes, selectively targeting and eliminating serotonergic neurons, much like how 6-hydroxydopamine is used to eradicate dopaminergic neurons.
In addition to its scientific applications, para-chloroamphetamine has been identified as a prospective designer drug. Another closely linked compound, 3-chloroamphetamine, is even more potent in releasing dopamine and serotonin, albeit with somewhat reduced neurotoxicity.
Furthermore, the N-methylated derivative, para-chloromethamphetamine (CMA), which metabolizes into para-chloroamphetamine in vivo, also exhibits neurotoxic properties.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 64-12-0 |
|---|---|
| PubChem CID | 3127 |
| IUPHAR/BPS | 4592 |
| ChemSpider | 3015 |
| UNII | 897NVD4A52 |
| ChEMBL | ChEMBL358967 |
| CompTox Dashboard (EPA) | DTXSID90897229 |
| Chemical and physical data | |
| Formula | C9H12ClN |
| Molar mass | 169.65 g·mol−1 |
Legal status
As of October 2015, China has classified 4-CA as a regulated substance.
FAQ
1. What is para-chloroamphetamine (PCA)?
Para-chloroamphetamine (PCA) is a chemical compound classified as a substituted amphetamine. It shares similarities with MDMA but is known for its substantially higher neurotoxicity.
2. What are the common names for PCA?
PCA is also referred to as 4-chloroamphetamine (4-CA) in scientific literature and discussions.
3. How does PCA affect the brain and body?
PCA acts as a monoamine releaser and influences the release of serotonin and dopamine. It results in stimulant effects similar to MDMA but with increased neurotoxicity.
4. Why do neurobiologists use PCA?
Neurobiologists use PCA as a neurotoxin to selectively destroy serotonergic neurons for research purposes, much like 6-hydroxydopamine is used to target dopaminergic neurons.
5. What are the risks and side effects associated with PCA use?
Using PCA can be risky due to its high neurotoxicity. It can lead to adverse effects such as potential damage to serotonin and dopamine systems, potentially impacting mood and behavior.
6. Is PCA a designer drug?
PCA has been detected as a designer drug, suggesting that it may be used recreationally. However, its high neurotoxicity makes it a risky choice for recreational use.
7. What is the difference between PCA and related compounds like 3-chloroamphetamine?
3-chloroamphetamine is a related compound that is even more potent in releasing dopamine and serotonin compared to PCA. However, it is slightly less neurotoxic. The choice between these compounds may depend on the specific research or recreational purposes.
8. Are there any other derivatives or compounds related to PCA?
Yes, there is a closely related N-methylated derivative called para-chloromethamphetamine (CMA). This compound metabolizes into PCA in vivo and also exhibits neurotoxic properties.
9. Is PCA legal in my country?
The legal status of PCA varies by country. It is essential to check your country’s specific regulations and laws regarding its possession, use, and distribution.
10. Where can I find more information about PCA?
For detailed and current information about PCA, you can refer to scientific literature, government regulations, and research publications in the fields of pharmacology and neuroscience. Always prioritize safety and adherence to relevant laws and regulations when dealing with substances like PCA.
References
- Investigating the Metabolism of para-Chloroamphetamine (PCA): Miller KJ, Anderholm DC, Ames MM (May 1986). “Biological Activation of para-Chloroamphetamine (PCA) into Chemically Reactive Intermediates by Hepatic and Brain Microsomes.” Biochemical Pharmacology. 35 (10): 1737–1742. doi:10.1016/0006-2952(86)90332-1. PMID 3707603.
- Insights into Cerebral Effects: Gal EM, Cristiansen PA, Yunger LM (January 1975). “Impact of p-Chloroamphetamine on Cerebral Tryptophan-5-Hydroxylase In Vivo: A Reevaluation.” Neuropharmacology. 14 (1): 31–39. doi:10.1016/0028-3908(75)90063-5. PMID 125387. S2CID 1068793.
- Effects on Serotonin Metabolism: Curzon G, Fernando JC, Marsden CA (August 1978). “5-Hydroxytryptamine: Consequences of Impaired Synthesis on Metabolism and Release in Rats.” British Journal of Pharmacology. 63 (4): 627–634. doi:10.1111/j.1476-5381.1978.tb17275.x. PMC 1668117. PMID 80243.
- Serotonin Loss and its Impact: Colado MI, Murray TK, Green AR (March 1993). “Serotonin Loss in Rat Brain Following Administration of 3,4-Methylenedioxymethamphetamine (MDMA), para-Chloroamphetamine, and Fenfluramine, and the Effects of Chlormethiazole and Dizocilpine.” British Journal of Pharmacology. 108 (3): 583–589. doi:10.1111/j.1476-5381.1993.tb12846.x. PMC 1908028. PMID 7682129.
- Cerebral Metabolic Responses: Freo U, Pietrini P, Pizzolato G, Furey M, Merico A, Ruggero S, et al. (November 1995). “Cerebral Metabolic Responses to Clomipramine Are Greatly Reduced Following Pre-treatment with the Specific Serotonin Neurotoxin para-Chloroamphetamine (PCA): A 2-Deoxyglucose Study in Rats.” Neuropsychopharmacology. 13 (3): 215–222. doi:10.1016/0893-133X(95)00053-G. PMID 8602894.
- Urine Sample Detection: Lin TC, Lin DL, Lua AC (May 2011). “Identification of para-Chloroamphetamine (PCA) in Urine Samples Using Mass Spectrometry.” Journal of Analytical Toxicology. 35 (4): 205–210. doi:10.1093/anatox/35.4.205. PMID 21513613.
- Impact on Brain Serotonin: Fuller RW, Schaffer RJ, Roush BW, Molloy BB (May 1972). “Influence of Drug Disposition on Brain Serotonin Reduction by Chloroamphetamines in Rats.” Biochemical Pharmacology. 21 (10): 1413–1417. doi:10.1016/0006-2952(72)90365-6. PMID 5029422.
- Behavioral Effects: Ogren SO, Ross SB (October 1977). “Substituted Amphetamine Derivatives. II. Behavioral Effects in Mice Related to Monoaminergic Neurons.” Acta Pharmacologica et Toxicologica. 41 (4): 353–368. doi:10.1111/j.1600-0773.1977.tb02674.x. PMID 303437.
- Influence on Dopamine Accumulation: Ross SB, Kelder D (May 1979). “Inhibition of 3H-Dopamine Accumulation in Reserpinized and Normal Rat Striatum.” Acta Pharmacologica et Toxicologica. 44 (5): 329–335. doi:10.1111/j.1600-0773.1979.tb02339.x. PMID 474143.
- Monoamine Effects: Fuller RW, Baker JC (November 1974). “Prolonged Reduction of Brain 5-Hydroxytryptamine Concentration by 3-Chloroamphetamine and 4-Chloroamphetamine in Iprindole-Treated Rats.” The Journal of Pharmacy and Pharmacology. 26 (11): 912–914. doi:10.1111/j.2042-7158.1974.tb09206.x. PMID 4156568. S2CID 41833796.
- Effects on Monoamine Uptake and Release: Ross SB, Ogren SO, Renyi AL (October 1977). “Substituted Amphetamine Derivatives. I. Influence on Uptake and Release of Biogenic Monoamines and on Monoamine Oxidase in the Mouse Brain.” Acta Pharmacologica et Toxicologica. 41 (4): 337–352. doi:10.1111/j.1600-0773.1977.tb02673.x. PMID 579062.
- Regulatory Update: “关于印发《非药用类麻醉药品和精神药品列管办法》的通知” (in Chinese). China Food and Drug Administration. 27 September 2015. Archived from the original on 1 October 2015. Retrieved 1 October 2015.