Summary
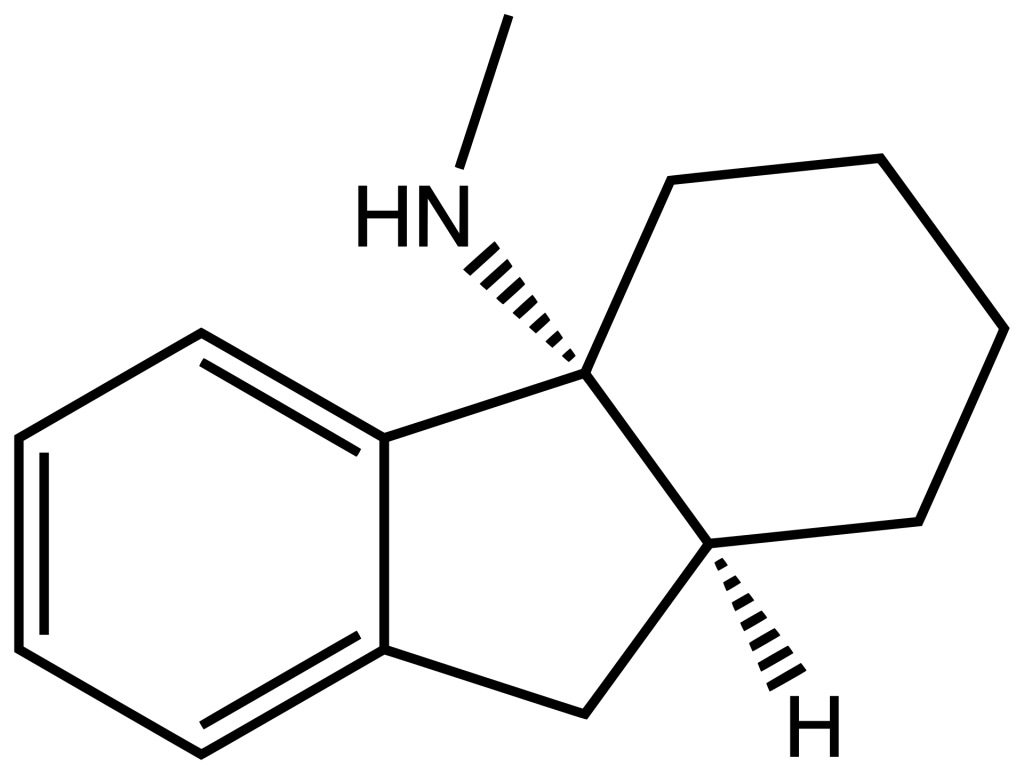
PD-137889, also known as N-methylhexahydrofluorenamine, is a chemical compound recognized for its potent activity as an NMDA receptor antagonist within the central nervous system. Its potency in this regard is approximately 30 times greater than the well-known representative of this class, ketamine. In animal studies, PD-137889 has demonstrated the ability to substitute for phencyclidine. Notably, when measuring its binding affinity ([3H]TCP[a] imperative), PD-137889 exhibits a Ki value of 27 nM, whereas ketamine’s Ki value is comparatively less potent at 860 nM.
| Identifiers | |
|---|---|
| 3D model (JSmol) | Interactive image |
| ChemSpider | 8279807 |
| PubChemCID | 10104280 |
| UNII | 2KU62QB3UM |
FAQ
1. What is PD-137889?
PD-137889 is a chemical compound known as N-methylhexahydrofluorenamine. It is notable for its decisive antagonistic action on NMDA receptors in the central nervous system.
2. What are NMDA receptors?
NMDA receptors, short for N-methyl-d-aspartate receptors, are a type of receptor in the brain that plays a crucial role in controlling synaptic plasticity, which is essential for learning and memory. Antagonists like PD-137889 can influence the activity of these receptors.
3. How potent is PD-137889 compared to ketamine?
PD-137889 is approximately 30 times more potent than ketamine, which is a well-known NMDA receptor antagonist. This increased potency makes it an attractive compound for scientific research.
4. What are the applications of PD-137889?
PD-137889 is primarily used in scientific research, especially in studies related to NMDA receptors and their functioning within the central nervous system. It is often employed as a research tool to understand various aspects of brain function and neurobiology.
5. How does PD-137889 affect the central nervous system?
As an NMDA receptor antagonist, PD-137889 blocks or modulates the activity of these receptors. This action can have a range of effects on neural signalling and synaptic plasticity, which makes it valuable for studying brain function and related disorders.
6. Can PD-137889 be used for medical or therapeutic purposes?
PD-137889 is not approved for medical or therapeutic use in humans. It is primarily a research compound and is not intended for clinical applications.
7. What are the potential risks and side effects of PD-137889?
Since PD-137889 is primarily a research tool, there needs to be more information available on its safety profile in humans. Researchers working with PD-137889 should take appropriate precautions to ensure safety.
8. Is PD-137889 available for purchase or personal use?
In most cases, PD-137889 is not available for purchase or personal use. It is typically distributed only to qualified researchers and institutions for scientific and laboratory purposes.
9. Where can I find more information about PD-137889?
For detailed information on PD-137889, you may refer to scientific literature research publications or contact laboratories and institutions involved in neurobiological research.
10. Can I participate in a study or clinical trial involving PD-137889?
Participation in studies involving PD-137889 would be subject to specific research projects conducted by scientific institutions. You may inquire with research institutions and universities that specialize in neuroscience and NMDA receptor studies for potential opportunities to participate in relevant research.
References
1. Hays, S.J., Novak, P.M., Ortwine, D.F., Bigge, C.F., Colbry, N.L., Johnson, G., Lescosky, L.J., Malone, T.C., Michael, A. (1993). Synthesis and pharmacological evaluation of hexahydrofluorenamines as noncompetitive antagonists at the N-methyl-D-aspartate receptor. This research article delves into the synthesis and evaluation of hexahydrofluorenamines as noncompetitive antagonists at NMDA receptors. It explores their potential pharmacological applications.
2. Nicholson, K.L., Balster, R.L. (2003). Evaluation of the phencyclidine-like discriminative stimulus effects of novel NMDA channel blockers in rats. This study examines the phencyclidine-like discriminative stimulus effects of novel NMDA channel blockers in rats, shedding light on their behavioral effects.
3. Bigge, C.F. (1993). Structural requirements for the development of potent N-methyl-D-aspartic acid (NMDA) receptor antagonists. Bigge’s work investigates the structural prerequisites for developing potent NMDA receptor antagonists, offering insights into the chemistry of these compounds.
4. Bigge, C.F., Malone, T.C. (1993). Overview: Agonists, Antagonists and Modulators of the N-methyl-D-aspartic acid (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropanoic acid (AMPA) Subtypes of Glutamate Receptors. This overview discusses various aspects of NMDA and AMPA receptor modulators, shedding light on their roles in glutamate receptor pharmacology.
5. Polycyclic amine derivatives useful as cerebrovascular agents. United States Patent; Coughenour, et al. Family ID: 22686445, Appl. #07/186,834. This patent focuses on polycyclic amine derivatives with potential applications as cerebrovascular agents, providing protection or treatment for certain cerebrovascular conditions.
6. [3H]N-[1-(2-thienyl)cyclohex-yl]piperidine. This appears to reference a compound or label, but additional context is required to provide a more detailed explanation.