Summary
QUCHIC (also known as BB-22, SGT-32, or 1-(cyclohexylmethyl)-1H-indole-3-carboxylic acid 8-quinolinyl ester) is a synthetic designer drug available through online vendors, marketed as a cannabimimetic agent. It initially surfaced in the artificial cannabis market in Japan in early 2013 and subsequently made its way to New Zealand.
QUCHIC’s structural composition suggests a comprehensive understanding of structure-activity relationships within the indole class of cannabimimetics, although the exact origins of its design remain unclear. Notably, QUCHIC, in conjunction with QUPIC, presents a distinct synthetic cannabinoid chemotype due to the presence of an ester linker at the indole 3-position, setting it apart from the familiar ketone structure found in JWH-018 and its analogues, or the amide linkage characteristic of compounds like SDB-001 and its derivatives.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1400742-42-8 |
|---|---|
| PubChem CID | 71711120 |
| ChemSpider | 29339967 |
| UNII | EUD4ZLB25R |
| CompTox Dashboard (EPA) | DTXSID40856801 |
| Chemical and physical data | |
| Formula | C25H24N2O2 |
| Molar mass | 384.479 g·mol−1 |
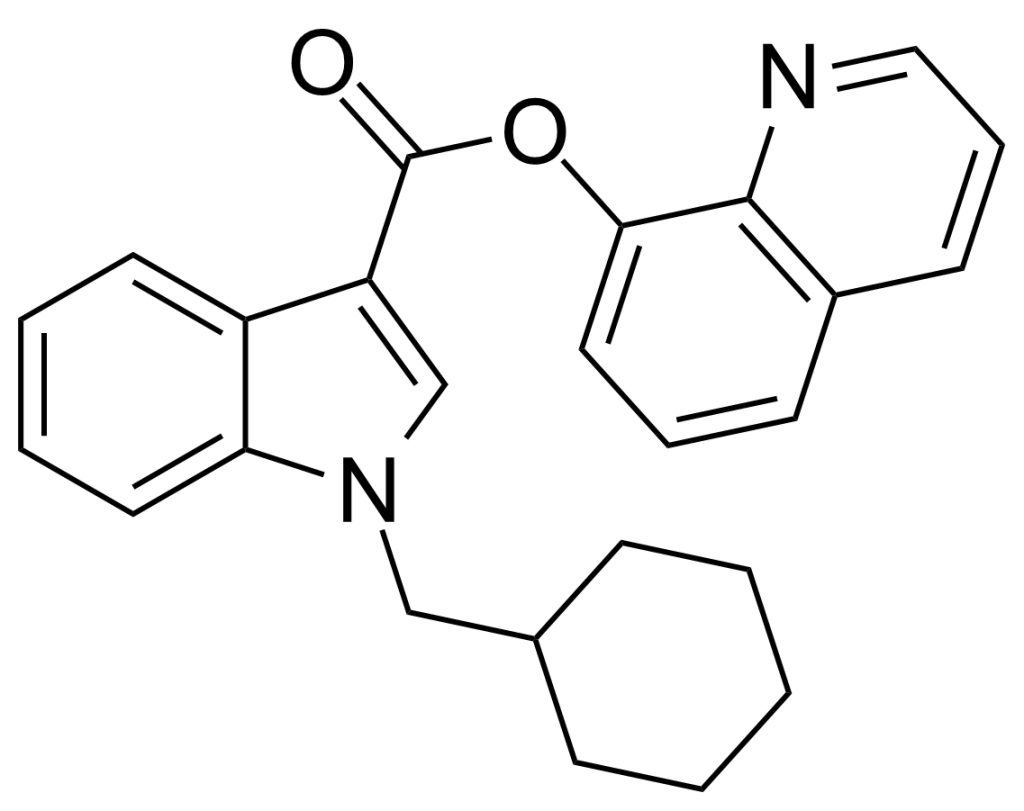
Pharmacology
BB-22 functions as a potent full agonist, exhibiting a remarkable binding affinity of 0.217nM at CB1 cannabinoid receptors and 0.338nM at CB2 cannabinoid receptors.
FAQ
1. What is QUCHIC?
- QUCHIC, also known as BB-22 or SGT-32, is a synthetic designer drug that is marketed as a cannabimimetic agent. It belongs to the class of synthetic cannabinoids designed to mimic the effects of natural cannabinoids found in marijuana.
2. When was QUCHIC first detected, and where?
- QUCHIC was first detected in synthetic cannabis products in Japan in early 2013. It subsequently appeared in the market in New Zealand. Its introduction marked its presence in the world of synthetic cannabinoids.
3. What sets QUCHIC apart from other synthetic cannabinoids?
- QUCHIC’s unique structural feature is its ester linker at the indole 3-position. This distinguishes it from other synthetic cannabinoids, such as JWH-018 and its analogues, which have a ketone structure, or compounds like SDB-001 and its analogues, characterized by an amide linkage.
4. Is QUCHIC safe for recreational use?
- The safety of QUCHIC for recreational use is uncertain. Like many synthetic cannabinoids, it carries potential health risks. Its use may lead to unpredictable effects and adverse reactions in individuals.
5. Is QUCHIC legal?
- The legal status of QUCHIC varies by country and region. It is important to check local laws and regulations regarding its legality. Many areas have implemented bans or restrictions on synthetic cannabinoids like QUCHIC.
6. Where can I find more information about QUCHIC and its effects?
- For detailed information about QUCHIC, its effects, legal status, and potential health risks, it is advisable to consult reputable sources, including government health agencies, substance abuse resources, and scientific literature. Stay informed with accurate and up-to-date information regarding this synthetic cannabinoid.
References
- Uchiyama N, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y (2013). “Discovery of Two Novel Cannabinoid Compounds: QUPIC and QUCHIC, and Identification of Two Emerging Cannabinoid Derivatives: ADB-FUBINACA and ADBICA, Along with Five Synthetic Cannabinoids, Detected Using α-PVT, a Thiophene Derivative, and AH-7921, an Opioid Receptor Agonist, in Illicit Substances.” Published in Forensic Toxicology, 31(2), 223–240. doi:10.1007/s11419-013-0182-9. S2CID 1279637.
- “New Zealand Government Bans Two Additional Substances Found in K2.” Press Release. Date: Tuesday, April 30, 2013.
- Hess C, Schoeder CT, Pillaiyar T, Madea B, Müller CE (2016). “Pharmacological Assessment of Synthetic Cannabinoids Detected as Components of Spice Mixtures.” Published in Forensic Toxicology, 34(2), 329–343. doi:10.1007/s11419-016-0320-2. PMC 4929166. PMID 27429655.