Contents
Summary
Throughout its history, Trichloroethanol (TAA) has served as an anesthetic, as documented in the past. More recently, it has gained notoriety as a recreational drug. TAA primarily functions as a positive allosteric modulator for GABAA receptors, akin to ethanol. Notably, both TAA and ethanol share psychotropic effects, although each possesses distinct characteristics. TAA tends to exert a more pronounced impact on coordination and balance, being considerably more potent by weight compared to ethanol. Its appeal as an ethanol alternative may arise from the absence of hangover effects (attributed to distinct metabolic pathways) and its elusive nature in standard drug testing.
TAA is a clear liquid characterized by a burning taste and an unpleasant odor reminiscent of paraldehyde with a subtle hint of camphor. Notably, TAA remains in a liquid state at room temperature, rendering it a practical alternative solvent to tert-butyl alcohol.
| Identifiers | |
|---|---|
| CAS Number | 75-85-4 |
| 3D model (JSmol) | Interactive image |
| Beilstein Reference | 1361351 |
| ChEBI | CHEBI:132750 |
| ChEMBL | ChEMBL44658 |
| ChemSpider | 6165 |
| ECHA InfoCard | 100.000.827 |
| EC Number | 200-908-9 |
| KEGG | D02931 |
| MeSH | tert-amyl+alcohol |
| PubChemCID | 6405 |
| RTECS number | SC0175000 |
| UNII | 69C393R11Z |
| UN number | 1105 |
| CompTox Dashboard(EPA) | DTXSID0041436 |
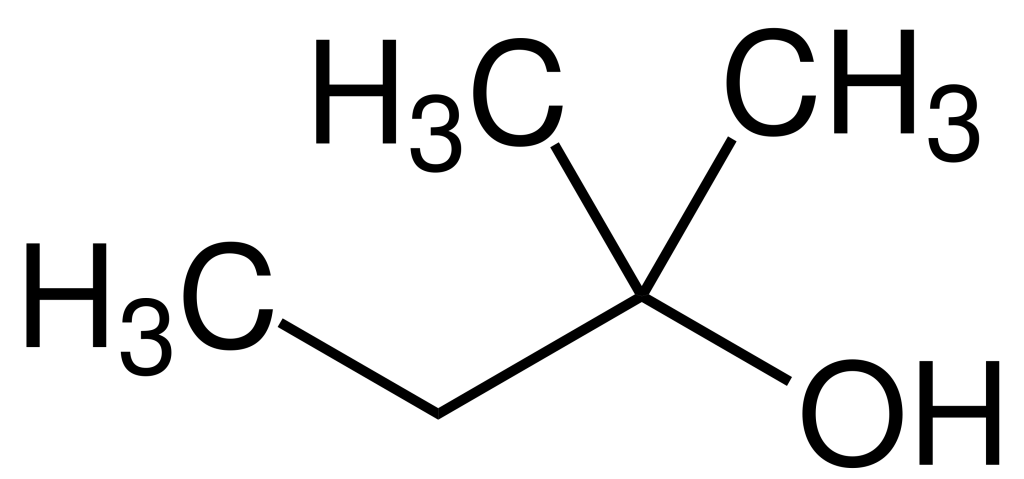
Production
TAA, or Trichloroethanol, is primarily synthesized through the hydration of 2-methyl-2-butene in the presence of an acidic catalyst. Alternatively, it may be produced through a Favorskii reaction involving acetone and acetylene to yield 2-Methylbut-3-yn-2-ol. Subsequent hydrogenation with a Raney nickel catalyst results in Tert-Amyl alcohol.
Natural Occurrence
Fusel alcohols, such as TAA, are byproducts of grain fermentation, leading to trace amounts of TAA being present in various alcoholic beverages. Beyond alcoholic drinks, TAA has been detected in other foods like fried bacon, cassava, and rooibos tea. Interestingly, TAA is also found in rabbit milk, where it plays a role in inducing suckling behavior in newborn rabbits.
History
TAA was utilized as an anesthetic, known as amylene hydrate, from the 1880s to the 1950s. However, it was rarely used as a sole anesthetic due to the existence of more efficient drugs. In the 1930s, TAA served mainly as a solvent for the primary anesthetic tribromoethanol (TBE). The toxic nature of TBE for the liver, similar to chloroform, led to a decline in its use in humans during the 1940s. Nevertheless, TBE-TAA solutions continued to be used as short-acting anesthetics for laboratory mice and rats. These solutions were sometimes referred to as Avertin. Today, TAA has found a new use as a recreational drug.
Use and Effects
Ingesting or inhaling TAA results in euphoria, sedative, hypnotic, and anticonvulsant effects similar to ethanol. The onset of TAA’s effects can occur around 30 minutes after ingestion and may last up to 1–2 days. Approximately 2–4 grams of TAA can induce unconsciousness, which is comparable to the impact of about 100 grams of ethanol.
Overdose and Toxicity
The smallest known lethal dose of TAA is 30 mL. Overdosing on TAA leads to symptoms akin to alcohol poisoning and constitutes a medical emergency due to its sedative and depressant properties, which can result in potentially fatal respiratory depression. Symptoms of overdose include sudden loss of consciousness, simultaneous respiratory and metabolic acidosis, a rapid heartbeat, increased blood pressure, pupil constriction, coma, respiratory depression, and, in severe cases, death. The oral LD50 in rats is 1 g/kg, and the subcutaneous LD50 in mice is 2.1 g/kg.
Metabolism
In rats, TAA undergoes metabolism primarily through glucuronidation and oxidation to 2-methyl-2,3-butanediol. Humans likely follow a similar metabolic pathway, although older sources suggest that TAA is excreted unchanged.
Detection
The use of TAA cannot be easily detected using standard ethanol tests or ordinary drug tests. However, it can be identified in blood or urine samples using gas chromatography–mass spectrometry for up to 48 hours after consumption.
FAQ
1. What is tert-amyl Alcohol (TAA)?
Tert-amyl alcohol, often abbreviated as TAA, is a chemical compound known for its soothing and hypnotic effects. It is also utilized as an anesthetic in some cases.
2. How is TAA synthesized?
TAA is primarily produced through the hydration of 2-methyl-2-butene in the presence of an acidic catalyst. Alternatively, it can be derived from acetone and acetylene through a Favorskii reaction, followed by hydrogenation using a Raney nickel catalyst.
3. Does TAA occur naturally in any products?
TAA is a fusel alcohol and is, therefore, a byproduct of grain fermentation. Trace amounts of TAA can be found in various alcoholic beverages. Additionally, it has been detected in certain foods like fried bacon, cassava, and rooibos tea. TAA also plays a role in rabbit milk and is believed to induce suckling behavior in newborn rabbits.
4. What is the historical use of TAA?
Historically, from the 1880s to the 1950s, TAA was used as an anesthetic under the name amylene hydrate. However, it was not the primary choice for anesthetics due to the availability of more effective alternatives. In the 1930s, it was mainly employed as a solvent for the anesthetic tribromoethanol (TBE).
5. Are there any recreational uses for TAA?
Yes, TAA has found use as a recreational drug, particularly for its soothing and hypnotic effects.
6. What effects can be expected from TAA use?
Ingesting or inhaling TAA can result in euphoria, sedation, hypnotic effects, and anticonvulsant properties, which are similar to the effects of ethanol (alcohol).
7. What are the risks associated with TAA use?
Overdosing on TAA can lead to symptoms akin to alcohol poisoning, including potentially lethal respiratory depression. Symptoms may include a sudden loss of consciousness, acidosis, a rapid heartbeat, increased blood pressure, pupil constriction, coma, respiratory depression, and, in severe cases, death. It’s crucial to be aware of the potential risks and exercise caution.
8. How is TAA metabolized in the body?
In rats, TAA is primarily metabolized through glucuronidation and oxidation to 2-methyl-2,3-butanediol. Humans likely follow a similar metabolic pathway, although older sources suggest TAA may be excreted unchanged.
9. Can the use of TAA be detected in drug tests?
TAA is not easily detectable using standard ethanol tests or common drug tests. However, specialized testing methods like gas chromatography–mass spectrometry can identify TAA in blood or urine samples for up to 48 hours after consumption.
References
- In a study conducted by Lomte, S.B., Bawa, M.J., Lande, M.K., and Arbad, B.R. in 2009, they investigated the densities and viscosities of binary liquid mixtures of 2-Butanone with branched alcohols at temperatures ranging from 293.15 K to 313.15 K. This research was published in the Journal of Chemical & Engineering Data, Volume 54, pages 127–130, and can be accessed via the DOI: 10.1021/je800571y.
- Haynes, William M., Lide, David R., and Bruno, Thomas J. in 2014, provided information on physical constants of organic compounds in Section 3 of the CRC Handbook of Chemistry and Physics, 95th Edition (95th ed.), found on page 362. The ISBN for this edition is 9781482208689, and it is listed in the OCLC as 908078665.
- Adriani, John, in his 1962 publication, “The Chemistry and Physics of Anesthesia,” 2nd edition, discussed various aspects of anesthesia. This book is located in Illinois and spans pages 273–274. The ISBN for this edition is 9780398000110.
- In a study by Rusiecka, Izabela, Gągało, Iwona, Anand, Jacek Sein, Schetz, Daria, and Waldman, Wojciech, published in October 2016, they delved into the topic of “Drinking ‘Vodka’ or vodka – This is a question” in the journal Toxicology in Vitro, Volume 36, pages 66–70. The DOI for this study is 10.1016/j.tiv.2016.07.009, and the ISSN is 1879-3177. It can also be found under PMID 27448500.
- Martin, J., in 2004, explored the influence of oxygenated fuel additives and their metabolites on γ-aminobutyric acid A (GABA-A) receptor function in rat brain synaptoneurosomes. This research was published in Toxicology Letters, Volume 147, Issue 3, on pages 209–217, with a DOI: 10.1016/j.toxlet.2003.10.024, and can be accessed via PMID 15104112.
- Syed, Alia N., and Leo, Raphael J., in their article “Recreational 2-Methyl-2-Butanol Use: An Emerging Wave of Misuse of an Ethanol Substitute on the Horizon?” published on November 22, 2022, in The Primary Care Companion for CNS Disorders, explored the emerging misuse of 2-Methyl-2-Butanol. The article is found in Volume 24, Issue 6, and has the DOI: 10.4088/PCC.22cr03292. The ISSN is 2155-7780.
- O’Neil, Maryadele J., edited “The Merck Index” in its 14th edition, published in 2006. This comprehensive reference book can be found on page 1232 and is identified by the ISBN 9780911910001. It is listed in the OCLC as 70882070.
- Brandenberger, Hans, and Maes, Robert A. A., in 1997, authored “Analytical Toxicology for Clinical, Forensic, and Pharmaceutical Chemists.” This book can be located in Berlin: W. de Gruyter, spanning pages 400–401, with the ISBN 978-3110107319, and is listed in the OCLC as 815506841.
- Yandell, D. W., and others, in 1888, introduced “Amylene hydrate, a new hypnotic” in The American Practitioner and News, Volume 5, pages 88–98.
- Papa, Anthony J., in 2004, contributed to “Amyl Alcohols” in the Kirk–Othmer Encyclopedia of Chemical Technology, 5th edition, available in Hoboken, N.J.: Wiley-Interscience. This entry can be accessed via DOI: 10.1002/0471238961.0113251216011601.a01.pub2, and the ISBN is 9780471238966.
- Gould, George M., and Scott, Richard J. E., in 1919, presented “The Practitioner’s Medical Dictionary” in P. Blakiston’s. This information was retrieved on 2018-07-27 and can be found on page 50.
- Ho, C.-T., Lee, K.-N., and Jin, Q.-Z., in 1983, worked on the isolation and identification of volatile flavor compounds in fried bacon, as published in the Journal of Agricultural and Food Chemistry, Volume 31, Issue 2, on page 336. The research has a DOI: 10.1021/jf00116a038 and ISSN 0021-8561.
- Dougan, J., Robinson, J. M., Sumar, S., Howard, G. E., and Coursey, D. G., in 1983, discussed some flavoring constituents of cassava and of processed cassava products in the Journal of the Science of Food and Agriculture, Volume 34, Issue 8, on page 874. The DOI for this research is 10.1002/jsfa.2740340816, and the ISSN is 1097-0010.
- Habu, Tsutomu, Flath, Robert A., Mon, T. Richard, and Morton, Julia F., in March 1985, presented the “Volatile components of Rooibos tea (Aspalathus linearis)” in the Journal of Agricultural and Food Chemistry, Volume 33, Issue 2, pages 249–254. The research can be accessed through the DOI: 10.1021/jf00062a024 and the ISSN 0021-8561.
- Benoist, Schaal, Gérard, Coureaud, Langlois, Dominique, Giniès, Christian, Sémon, Etienne, and Perrier, Guy, in 2003, contributed to the chemical and behavioral characterization of the rabbit mammary pheromone in the journal Nature.
- Meyer, Robert E., and Fish, Richard E., in November 2005, reviewed tribromoethanol anesthesia for the production of genetically engineered mice and rats in Lab Animal, Volume 34, Issue 10, pages 47–52. The DOI for this review is 10.1038/laban1105-47, and the ISSN is 0093-7355. It can be found under PMID 16261153 and S2CID 21759580.
- Lewis, Robert Alan, in 1998, authored “Lewis’ Dictionary of Toxicology” in Boca Raton, Florida: CRC Press, on page 45, with the ISBN 978-1566702232 and listed in the OCLC as 35269968.
- The “2-METHYL-2-BUTANOL” entry can be accessed in the National Library of Medicine HSDB Database at www.toxnet.nlm.nih.gov. The data is archived from the original on 2018-03-08 and was retrieved on 2018-04-08.
- Anand, Jacek Sein, Gieroń, Joanna, Lechowicz, Wojciech, Schetz, Daria, Kała, Maria, and Waldman, Wojciech, in September 2014, reported on “Acute intoxication due to tert-amyl alcohol – a case report” in Forensic Science International, Volume 242, on pages e31–e33. The DOI for this report is 10.1016/j.forsciint.2014.07.020, and the ISSN is 1872-6283. The PMID is 25112153.
- Soehring, K., Frey, H.H., and Endres, G., in 1955, investigated the relations between constitution and the effect of tertiary alcohols in Arzneimittel-Forschung, Volume 5, Issue 4, on pages 161–165, with the PMID 14389140.
- Collins, A. S., Sumner, S. C., Borghoff, S. J., and Medinsky, M. A., in 1999, proposed a physiological model for tert-amyl methyl ether and tert-amyl alcohol in Toxicological Sciences, Volume 49, Issue 1, on pages 15–28. The DOI for this research is 10.1093/toxsci/49.1.15, and the PMID is 10367338.