Summary
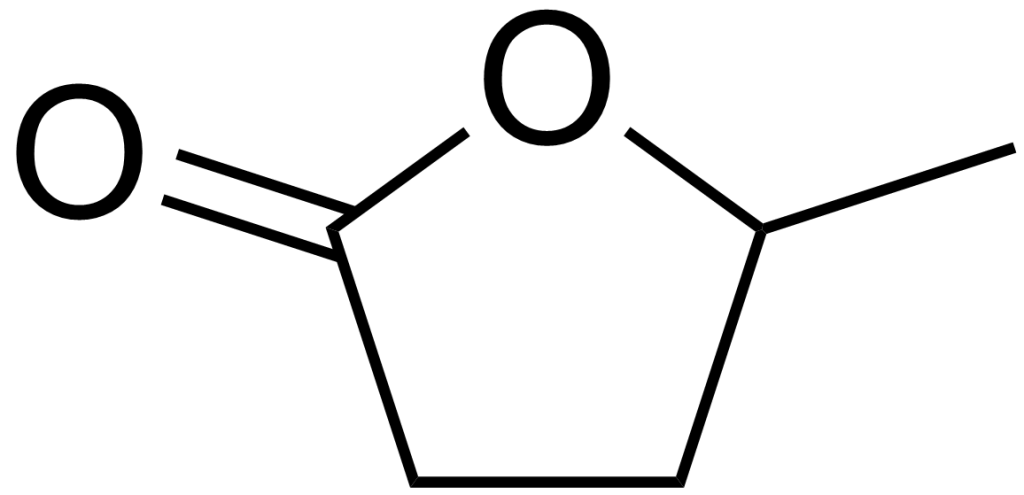
γ-Valerolactone, often abbreviated as GVL, represents an organic compound characterized by the chemical formula C5H8O2. This clear, colourless liquid ranks among the more prevalent lactones. Although GVL possesses chirality, it is commonly employed in its racemic form. It can be readily derived from cellulosic biomass and holds promise as both a potential fuel source and an environmentally friendly solvent.
GVL acts as a prodrug for γ-hydroxyvaleric acid (GHV), a substance with effects similar to those of γ-hydroxybutyric acid (GHB), albeit with somewhat reduced potency. Due to the regulatory controls placed on GHB in numerous regions worldwide, GVL has gained traction as a lawful alternative to GHB.
| Identifiers | |
|---|---|
| CAS Number | 108-29-2 |
| 3D model (JSmol) | Interactive image |
| Beilstein Reference | 80420 |
| ChEBI | CHEBI:48569 |
| ChEMBL | ChEMBL195593 |
| ChemSpider | 7633 |
| ECHA InfoCard | 100.003.245 |
| EC Number | 203-569-5 |
| PubChemCID | 7921 |
| UNII | O7056XK37X |
| UN number | 1224 |
| CompTox Dashboard(EPA) | DTXSID0047618 |
Synthesis
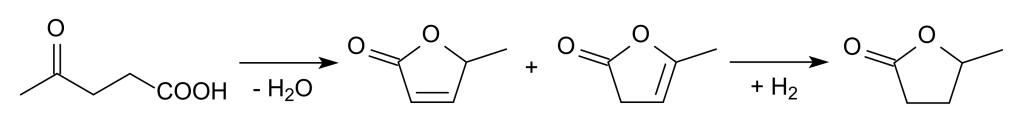
GVL is manufactured from levulinic acid, a compound sourced from hexoses. In a standard procedure, cellulosic biomass, like corn stover, sawgrass, or wood, undergoes hydrolysis, facilitated by acid catalysts, which break it down into glucose and other sugars. The resulting glucose can subsequently be dehydrated, yielding formic acid and levulinic acid through hydroxymethylfurfural conversion. Levulinic acid then undergoes cyclization to form intermediate unsaturated ring compounds, which can be further hydrogenated to produce gamma-valerolactone. This compound holds promise for various applications, including its potential use as a liquid fuel.
Potential applications
GVL has emerged as a promising eco-friendly solvent, prized for its natural aroma, and finds applications in the perfume and flavour industries. Notably, it stands as a structural isomer of δ-valerolactone.
Potential as a Fuel Source
Due to its accessibility from glucose, GVL has long been hailed as a prospective “green fuel.” GVL retains a remarkable 97% of the energy contained in glucose and can be seamlessly blended into gasoline, delivering performance on par with ethanol/gasoline blends. To optimize its utilization in conventional combustion engines, it may be more efficient to convert GVL into liquid alkenes or alkanes. This transformation commences with the ring-opening of GVL, yielding a blend of pentanoic acids, which can be subsequently decarboxylated to produce butene and CO2, utilizing zeolite catalysts. Following dehydration, these products can be oligomerized at elevated pressures in the presence of common acid catalysts, yielding alkenes with higher molecular weights, tailor-made for gasoline and other fuel applications.
One of GVL’s standout advantages as a biofuel lies in its cost-effectiveness, as it can be produced economically, utilizing readily available feedstocks, with prices ranging from 2 to 3 US dollars per gallon. The transition of GVL into transportation fuel-ready alkenes necessitates a streamlined system comprising two flow reactors, two-phase separators, and a simple pumping arrangement for aqueous GVL feed delivery. Furthermore, the absence of precious metal catalysts in this process contributes to a reduction in the overall cost of fuel production.
Potential in Biomass-Derived Fuel Production
Beyond its role as a standalone fuel, gamma-valerolactone has exhibited promise in laboratory-scale thermocatalytic production of soluble carbohydrates sourced from corn stover and wood, yielding high yields. In this method, biomass undergoes reactions in a solvent mixture consisting of water, dilute sulfuric acid, and gamma-valerolactone derived from biomass. Gamma-valerolactone facilitates thermocatalytic hydrolysis by thoroughly solubilizing the raw materials, including lignins. The saccharide products can be recovered from the lactone into a water solution by introducing salt or liquid carbon dioxide as antisolvents. These saccharides can serve as a feedstock for the production of furans or ethanol, yielding high returns, while the gamma-valerolactone is recycled back into the catalytic cycle.
Membrane Fabrication
Gamma-valerolactone has garnered attention for its potential in the preparation of polymer membranes. Traditional solvents, often toxic, have prompted exploration into green solvents in recent years. Due to its eco-friendly attributes, Gamma-Valerolactone has displayed promise in the production of polysulfone membranes as a co-solvent, contributing to the development of safer and more sustainable membrane fabrication processes.
FAQ
1. What is γ-Valerolactone (GVL)?
γ-Valerolactone, commonly referred to as GVL, is an organic compound with the chemical formula C5H8O2. It is a colourless liquid and is categorized as one of the more common lactones.
2. Where is GVL commonly found or sourced from?
GVL is often derived from glucose, which can be obtained from cellulosic biomass sources such as corn stover, sawgrass, or wood. It is a product of various chemical reactions and processes involving biomass feedstocks.
3. What are the primary applications of GVL?
GVL has versatile applications. It is used as a green solvent, and its herbal aroma makes it valuable in the perfume and flavour industries. Additionally, it is explored as a potential fuel source and serves as a valuable precursor for the production of various chemicals.
4. How is GVL utilized as a potential fuel?
GVL is considered a “green fuel” because it retains a significant portion of the energy contained in glucose. It can be blended with gasoline and used as a transportation fuel. It can also be converted into liquid alkenes or alkanes, which can serve as alternatives to traditional gasoline or diesel.
5. What are the advantages of GVL as a biofuel?
GVL’s cost-effectiveness is a crucial advantage, as it can be produced from readily available feedstocks at an economical price. Additionally, the production process does not require precious metal catalysts, further reducing production costs.
6. How is GVL involved in the production of biomass-derived fuels?
GVL plays a crucial role in the thermocatalytic production of soluble carbohydrates from biomass sources like corn stover and wood. It promotes the hydrolysis of biomass into monosaccharides and allows for the production of furans or ethanol, while GVL can be recycled in the catalytic cycle.
7. What is GVL’s role in membrane fabrication?
GVL is explored for its potential in the creation of polymer membranes, primarily due to its environmentally friendly profile. It can be used as a co-solvent in membrane fabrication processes, replacing traditional, often toxic solvents.
8. Is GVL widely available and regulated?
GVL is relatively accessible and not subject to stringent regulations in the same way some other chemicals are. It is a valuable compound with potential applications across various industries.
9. Are there safety considerations for handling GVL?
Like any chemical substance, GVL should be handled with care. Users should follow standard safety protocols when working with GVL, including wearing appropriate protective equipment and ensuring proper ventilation.
10. How is GVL different from other similar compounds?
GVL is a structural isomer of δ-valerolactone, and it offers unique properties and applications compared to other compounds, making it a subject of ongoing research and development.
References
- The National Institutes of Health (NIH) National Toxicology Program delves into the study of GVL’s properties and potential applications in various fields.
- Research by Baird, Zachariah Steven, et al., published in the “International Journal of Thermophysics” (Nov 6, 2019), offers insights into GVL’s vapor pressures, densities, and PC-SAFT parameters for 11 bio-compounds.
- Gain knowledge on GVL’s classification and labeling with the “Summary of Classification and Labeling” retrieved on December 5, 2021.
- Andresen-Streichert H, et al., in their work published in the “Journal of Analytical Toxicology,” investigate the uptake of gamma-valerolactone and the detection of gamma-hydroxyvaleric acid in human urine samples.
- Delve into the world of forensic drug analysis with Fred Smith’s “Handbook of Forensic Drug Analysis,” published by Academic Press on December 31, 2004.
- In the “Chemical Reviews” (2006), Huber, George W., et al., explore the synthesis of transportation fuels from biomass, delving into the chemistry, catalysts, and engineering involved.
- Huber, G. W., et al., in the “Angewandte Chemie International Edition” (2007), discuss synergies between bio- and oil refineries for the production of fuels from biomass.
- The potential of GVL as an ideal biofuel is highlighted in Neil Savage’s article “Fuel Options: The Ideal Biofuel” in the journal “Nature” (2011).
- Horváth, I. T., et al., emphasize GVL as a sustainable liquid for energy and carbon-based chemicals in “Green Chemistry” (2008).
- Bond, Jesse Q., et al., in their research published in “Science” (2010), detail the integrated catalytic conversion of γ-Valerolactone to liquid alkenes for transportation fuels.
- Explore the possibilities of oligomerization of isobutene on sulfated titania in the “Catalysis Today” (2005) article by Mantilla, A., et al.
- Luterbacher, Jeremy S., et al., present nonenzymatic sugar production from biomass using GVL in “Science” (2014).
- Figoli, A., et al., discuss non-toxic solvents for membrane preparation in “Green Chemistry” (2014), aiming to make membrane fabrication more environmentally friendly.
- Dong, Xiaobo, et al., investigate the use of GVL in “Green Polymer Chemistry” (2018) as a solvent for polysulfone membrane fabrication, exploring its potential in creating eco-friendly membranes.