Summary
Butane-1,4-diol, distinct from 1,3-butanediol, represents primary alcohol and an organic compound identified by the chemical formula HOCH2CH2CH2CH2OH. This substance is a clear and thick liquid, comprising one of the four enduring isomeric forms of butanediol.
Synthesis
In the realm of industrial chemical synthesis, acetylene undergoes a reaction with two portions of formaldehyde to yield butyne-1,4-diol. The subsequent hydrogenation of butyne-1,4-diol results in the formation of butane-1,4-diol. Furthermore, this compound can be manufactured on a large scale using the Davy process, where maleic anhydride is initially transformed into the methyl maleate ester and subsequently hydrogenated. Additional pathways for its production include starting from butadiene, allyl acetate, and succinic acid.
Industrial use
Butane-1,4-diol finds significant industrial application as a solvent and plays a vital role in the production of specific types of plastics, elastic fibers, and polyurethanes. In the field of organic chemistry, 1,4-butanediol serves as a crucial precursor for synthesizing γ-butyrolactone (GBL). Under the influence of phosphoric acid at elevated temperatures, it undergoes dehydration to yield the valuable solvent known as tetrahydrofuran.[7] Additionally, when subjected to soluble ruthenium catalysts at approximately 200°C, the diol experiences dehydrogenation, resulting in the formation of butyrolactone. It is also employed in the synthesis of 1,4-Butanediol diglycidyl ether, a substance used as a reactive diluent for epoxy resins.
Global production statistics for butane-1,4-diol were estimated at around one million metric tons annually, with a market price of approximately US$2,000 (€1,600) per ton as of 2005. By 2013, worldwide production had reportedly reached billions of pounds, consistent with an annual output of approximately one million metric tons.
A substantial portion of this production is directed toward the dehydration process to generate tetrahydrofuran, particularly for the manufacturing of fibres such as Spandex. BASF stands as the largest producer in this industry.
Use as a recreational drug
Butane-1,4-diol is also occasionally employed as a recreational substance, recognized by some users under names such as “One Comma Four,” “Liquid Fantasy,” “One Four Bee,” or “One Four B-D-O.” It’s worth noting that its effects have been a subject of legal debate in various Federal Courts. Some courts have contended that 1,4-butanediol induces effects akin to those of gamma-hydroxybutyrate (GHB), which is a metabolic byproduct of 1,4-butanediol. Conversely, other Federal courts have concluded otherwise.
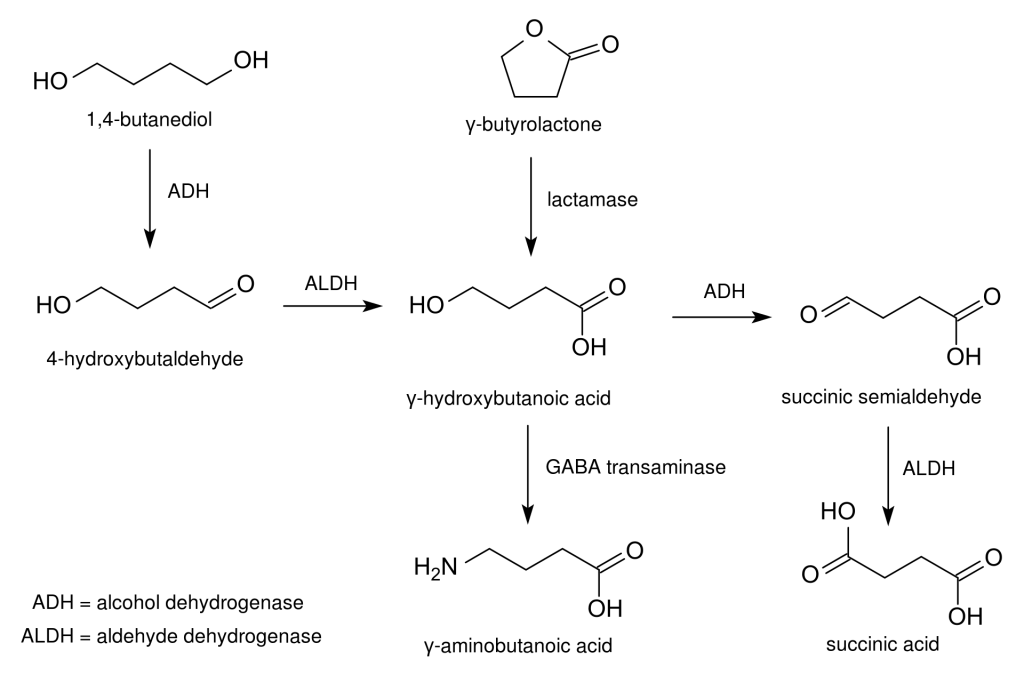
In terms of its pharmacokinetics, butane-1,4-diol undergoes rapid conversion into gamma-hydroxybutyric acid, facilitated by the enzymes alcohol dehydrogenase and aldehyde dehydrogenase. Variations in the levels of these enzymes among individuals may account for differences in the effects and side effects experienced by users. Notably, the simultaneous use of ethanol and GHB already carries substantial risks, and combining ethanol with 1,4-butanediol can result in significant interactions and numerous potential hazards. This is due to the fact that the same enzymes responsible for metabolizing alcohol are also involved in the metabolism of 1,4-butanediol, raising the risk of a dangerous drug interaction. Emergency room patients who overdose on both ethanol and 1,4-butanediol often initially present symptoms of alcohol intoxication. As ethanol is metabolized, 1,4-butanediol can then more effectively compete for these enzymes, leading to a subsequent period of intoxication as it converts into GHB.
Pharmacodynamics
Butane-1,4-diol appears to exhibit two distinct types of pharmacological actions. The primary psychoactive effects of 1,4-butanediol arise from its metabolic conversion into GHB, although a study has suggested that 1,4-butanediol might possess alcohol-like pharmacological effects in its own right. This hypothesis is based on findings that coadministration of butane-1,4-diol with ethanol resulted in the potentiation of specific behavioural effects of ethanol. However, it’s important to note that this potentiation of ethanol’s effects may stem from competition for the alcohol dehydrogenase and aldehyde dehydrogenase enzymes with concurrently administered 1,4-butanediol. This shared, rate-limiting metabolic pathway consequently leads to reduced metabolism and clearance for both compounds, including ethanol’s well-known toxic metabolite, acetaldehyde.
Another study involving intracerebroventricular injection of butane-1,4-diol in rats found no discernible effect, contradicting the notion that butane-1,4-diol possesses inherent alcohol-like pharmacological effects.
Much like gamma-hydroxybutyric acid, butane-1,4-diol is safe when used in small amounts. However, higher doses can result in adverse effects, including nausea, vomiting, dizziness, sedation, vertigo, and, in extreme cases, death if ingested in substantial quantities. When combined with alcohol, anxiolytic effects are diminished, and side effects are heightened.
Legality
While butane-1,4-diol is not currently federally scheduled in the United States, several states have classified 1,4-butanediol as a controlled substance. Some individuals have faced legal prosecution for the possession of 1,4-butanediol under the Federal Analog Act on the grounds that it is substantially similar to GHB. In 2002, a federal case in New York ruled that 1,4-butanediol couldn’t be considered an analogue of GHB under federal law, but this decision was later overturned by the Second Circuit. A jury in the Federal District Court in Chicago found that 1,4-butanediol was not an analogue of GHB under federal law. This finding was not contested in the case’s appeal to the Seventh Circuit Court of Appeals, although it did not impact the outcome of the case.[20] In the United Kingdom, 1,4-butanediol was scheduled in December 2009 (along with another GHB precursor, gamma-butyrolactone) as a Class C controlled substance. In Germany, the substance is not explicitly illegal but may be treated as such if used as a drug. It is regulated as a Schedule VI precursor in Canada.
2007 Contamination of Bindeez Toy
Additionally, in November 2007, a toy known as “Bindeez” (called “Aqua Dots” in North America) was recalled by the distributor due to the presence of butane-1,4-diol. This toy consisted of tiny beads that adhered to each other when exposed to water. The substitution of less toxic pentane-1,5-diol with butane-1,4-diol was suspected to be an attempt by the production plant to reduce costs. The presence of butane-1,4-diol was identified using GC-MS. The price of butane-1,4-diol was notably lower, ranging from approximately US$1,350 to $2,800 per metric ton, whereas 1,5-pentanediol was priced at about US$9,700 per metric ton.
2021 Poisoning at Darmstadt Technical University
In August 2021, a group of individuals fell severely ill after consuming beverages at building L2.01 at the Lichtwiese Campus of Darmstadt Technical University in Germany. Seven people exhibited severe symptoms, with two requiring transportation to a hospital in Frankfurt am Main and one person, aged 30, being in critical condition for a period. The presence of butane-1,4-diol was detected in milk packages and water filters at the location. Additionally, detectives found bromophenols and dicyclohexylamine at the scene.
FAQ
- What is 1,4-Butanediol (1,4-BDO)?
- 1,4-Butanediol is a chemical compound commonly used for industrial and pharmaceutical purposes. It can be found as a colourless, odourless liquid and is a precursor to gamma-hydroxybutyrate (GHB), which is known for its psychoactive effects.
- What are the industrial applications of 1,4-butanediol?
- 1,4-Butanediol is used as a solvent in various industries and plays a role in the production of plastics, elastic fibers, and polyurethanes.
- Is 1,4-butanediol used recreationally?
- Yes, 1,4-Butanediol has been used recreationally by some individuals under various street names like “One Comma Four” and “Liquid Fantasy.” It is known for its potential psychoactive effects, primarily when metabolized into GHB.
- What are the potential effects of 1,4-Butanediol use?
- The effects of 1,4-Butanediol use are similar to GHB and may include feelings of euphoria, relaxation, and disinhibition. However, it can also lead to side effects such as nausea, vomiting, dizziness, and sedation.
- Is 1,4-Butanediol safe to use?
- While 1,4-Butanediol can produce euphoric effects, it is not considered safe, mainly when used recreationally or in large amounts. Higher doses can lead to adverse reactions, including potentially life-threatening situations.
- What is the relationship between 1,4-Butanediol and GHB?
- 1,4-Butanediol is a precursor to GHB and is converted into GHB within the body. Both substances can produce similar psychoactive effects.
- Are there any risks associated with co-administering 1,4-Butanediol and alcohol?
- Yes, co-administration of 1,4-butanediol and alcohol can be dangerous. Both substances are metabolized by the same enzymes, leading to a risk of dangerous drug interactions, including prolonged intoxication.
- Is 1,4-Butanediol legal?
- The legal status of 1,4-Butanediol varies by country and region. In the United States, it is not federally scheduled, but some states have classified it as a controlled substance. In other countries, it may be controlled or treated as illegal if used recreationally.
- Why was 1,4-Butanediol recalled in the context of a toy in 2007?
- In 2007, a toy called “Bindeez” was recalled due to the presence of 1,4-Butanediol. The production plant seemingly used 1,4-butanediol as a substitute for a more expensive, less toxic chemical in an attempt to reduce production costs.
- What happened in the 2021 poisoning incident at Darmstadt Technical University?
- In 2021, a group of individuals became seriously ill after consuming beverages at Darmstadt Technical University. The incident was linked to the presence of 1,4-butanediol in milk packages and water filters at the location, along with other potentially harmful substances.
References
- Chemical Synthesis: 1,4-Butanediol is utilized as a key intermediate chemical in the production of other compounds, including butanediol-based polymers and solvents.
- Polymer Production: It is used to create polybutylene terephthalate (PBT) and other engineering plastics, which have applications in the automotive and electrical industries.
- Solvent: 1,4-Butanediol serves as a solvent in the manufacturing of various products, such as inks, perfumes, and cleaning agents.
- Recreational Use: Unfortunately, 1,4-Butanediol has also been misused as a recreational drug due to its euphoric and sedative effects. However, it is important to note that such use can be dangerous and is often subject to legal restrictions.
- Epoxy Resin Modification: It is employed as a diluent and viscosity modifier in the production of epoxy resins, enhancing their performance in various applications.
- Bio-Based Production: Recent developments have explored the bio-based production of 1,4-Butanediol, which can have environmental benefits.