Contents
Summary
Gamma-butyrolactone (GBL), also known as γ-butyrolactone, is a colourless liquid that readily absorbs moisture from the air and has a faint, distinct odour. This compound represents the simplest form of a four-carbon lactone. Its primary application lies in serving as an intermediate compound for the synthesis of various other chemicals, including methyl-2-pyrrolidone.
In the context of human physiology, GBL functions as a prodrug for gamma-hydroxybutyric acid (GHB). Additionally, it is commonly employed for recreational purposes. GBL is recognized for its ability to act as a central nervous system (CNS) depressant, inducing effects akin to those of barbiturates.
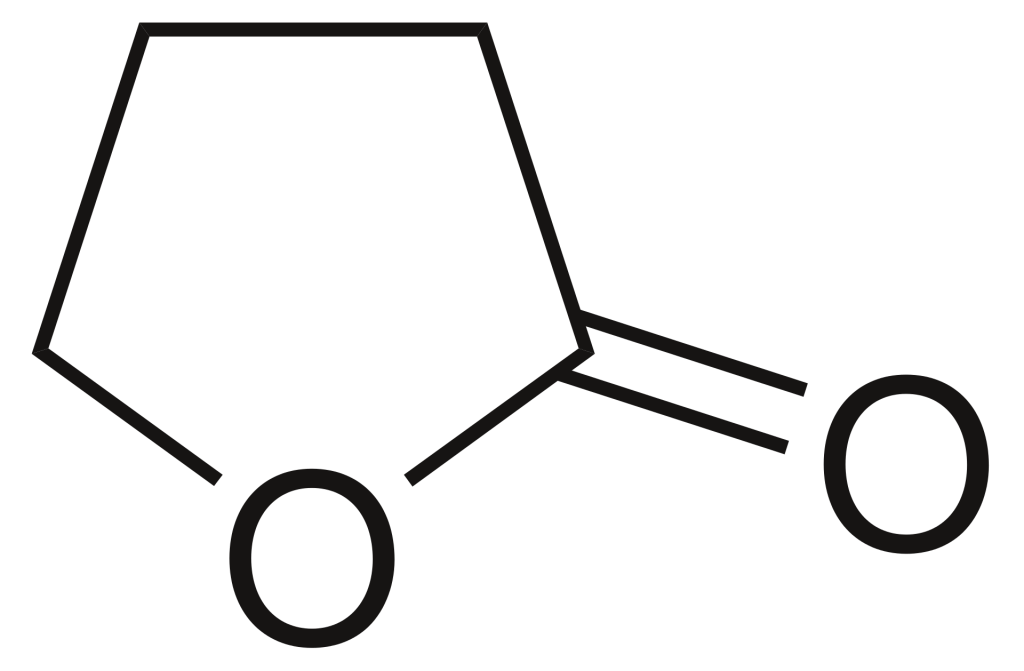
Occurrence
GBL has been identified in extractions from samples of pure, unaltered wines. This discovery suggests that GBL is a component that occurs naturally in certain wines and might also be found in comparable items. The measured concentration was approximately 5 micrograms per millilitre (5 μg/mL) and was readily discernible through a straightforward extraction method followed by GC/MS analysis. GBL can also be present in cheese flavourings, although it typically contributes to a content of 0.0002% GBL in the end product.
Production and synthesis
The industrial production of γ-Butyrolactone involves the dehydrogenation of 1,4-butanediol, carried out at a temperature ranging from 180 to 300 degrees Celsius under atmospheric pressure. This process takes place in the presence of a copper catalyst.
The overall yield of this industrial process is approximately 95%. To achieve purification, a liquid-gas-phase extraction method is employed.
In laboratory settings, an alternative method for obtaining γ-Butyrolactone is through the oxidation of tetrahydrofuran (THF), for instance, using aqueous sodium bromate. Another viable route involves the transformation of GABA, with a diazonium intermediate as an intermediate step.
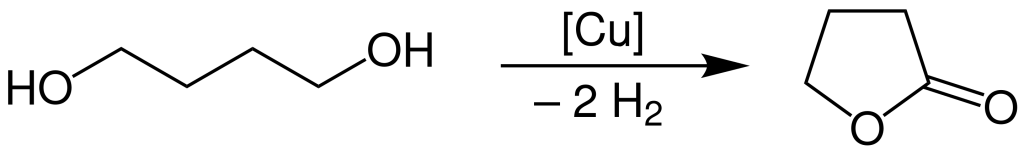
Reactions
In primary conditions, such as when immersed in a sodium hydroxide solution, γ-Butyrolactone (GBL) undergoes hydrolysis, resulting in the formation of sodium gamma-hydroxybutyrate, which is the sodium salt of gamma-hydroxybutyric acid. In an acidic aqueous environment, an equilibrium exists between the GBL lactone and its corresponding acid form. These compounds can subsequently contribute to the formation of the polymer poly(4-hydroxybutyrate) and the dimer 1,6-Dioxecane-2,7-dione. When subjected to a non-nucleophilic base like lithium diisopropylamide, GBL experiences deprotonation at the alpha carbon atom adjacent to the carbonyl group. A related compound, caprolactone, can be employed to create a polyester using a similar process.
Polymerization: The ring-opening polymerization of butyrolactone yields polybutyrolactone. This polymer can revert to its monomeric form through thermal cracking. It is claimed that poly(GBL) is a competitive alternative to the commercial biomaterial poly(4-hydroxybutyrate), or P4HB. Furthermore, it is suggested that poly(GBL) is more cost-effective to produce than P4HB despite both being derived from biological sources.
Uses
Butyrolactone serves primarily as a precursor for various other chemicals. When reacted with methylamine, it produces NMP, and with ammonia, it yields pyrrolidone. It is also utilized as a solvent in the formulation of lotions and specific polymers. Additionally, it is a starting material for the herbicide 2-methyl-4-chlorophenoxybutyric acid. In the realm of electrolytic capacitors, GBL is used as an organic solvent due to its wide liquid range, chemical stability, and high dielectric constant. Often, it is combined with a small proportion of ethylene glycol, commonly in a 9:1 ratio, to adjust internal resistivity. Furthermore, it finds applications as a solvent in various laboratory experiments, such as the preparation of methylammonium lead halide.
Pharmacology: GBL itself is not pharmacologically active; its mechanism of action stems from its role as a prodrug for GHB (gamma-hydroxybutyrate).
Pharmacokinetics: GBL is rapidly transformed into GHB by paraoxonase (lactonase) enzymes present in the bloodstream. Animals lacking these enzymes do not exhibit any effects from GBL. GBL is more lipophilic (fat-soluble) than GHB, resulting in faster absorption and higher bioavailability. These pharmacokinetic differences make GBL tend to be more potent and faster-acting than GHB, albeit with a shorter duration. In comparison, the related compound 1,4-butanediol (1,4-B) tends to be slightly less potent, slower to take effect, but longer-acting than GHB.
Nutritional Supplement
Gamma-butyrolactone (GBL) has gained attention for its potential to increase the secretion of sleep-related growth hormone (GH) as it serves as a prodrug of GHB. In response to the scheduling of GHB, GBL was marketed as a nutritional supplement under the names Revivarant and Renewtrient until they were prohibited by the FDA.
Recreational Drug
GBL is a prodrug of GHB, and its recreational use is entirely contingent on this property. GBL overdose can lead to irrational behaviour, severe illness, comatose states, and even fatality. To circumvent GHB restriction laws, home synthesis kits were introduced to convert GBL and 1,4-B into GHB. GBL possesses a unique taste and odour, often described as reminiscent of stale water, synthetic melon fragrance, or burnt plastic. This is distinct from GHB, which is characterized by a “salty” taste.
Due to its relatively uncomplicated synthesis, GBL has gained popularity among young people in French nightclubs.
Dangers
When ingested undiluted, GBL can irritate the oesophagus and gastrointestinal tract, potentially resulting in nausea and other related issues. GHB, for which GBL is a precursor, exhibits biphasic effects, with euphoria at lower doses and sedation at higher doses. The soothing effect can lead to unconsciousness, especially when combined with alcohol, which significantly increases the risk of vomiting and fatality. Numerous harm reduction organizations strongly advise against combining these two substances.
Reports have documented deaths associated with GBL, often in conjunction with alcohol or other depressants. GBL has also gained notoriety as a substance sometimes employed as a date rape drug.
Addictiveness and Dependence
Frequent GHB or GBL use, even in moderate, long-term doses, typically does not result in substantial physical dependence for most users. Quitting or abstaining from use is usually achievable with minimal difficulty. However, heavy and frequent consumption can lead to physical and psychological dependence. Users may adopt a ’24/7′ dosing regimen to avoid withdrawal symptoms, mainly if they have developed a tolerance.
Withdrawal symptoms, when they occur, seem to be dosage and duration-dependent. Light to moderate users may experience sleep-related issues, while heavy, prolonged use can lead to severe withdrawal symptoms similar to those seen in Benzodiazepine withdrawal syndrome (BWS).
Dose
Pure GBL, when metabolized, is equivalent to 1.65 grams of sodium gamma-hydroxybutyrate (NaGHB), the standard form. Therefore, doses are typically measured in single millilitres, either consumed all at once or sipped throughout the evening.
Legal Status
The legal status of GBL varies by country:
- Australia: GBL is classified as a health-endangering substance, and there are penalties for possession, sale, or driving under its influence.
- Canada: GBL is a Controlled Substance under Schedule VI of the Controlled Drugs and Substances Act, with strict regulations on its import and export.
- Germany: Possession of GBL is not illegal, but its distribution is controlled and may be punishable when intended for human consumption or GHB synthesis.
- Hong Kong SAR: GBL is classified as a dangerous drug under the Dangerous Drugs Ordinance, with stringent penalties for unlawful possession.
- Israel: GBL has been classified as an illegal substance.
- Netherlands: Possession is not illegal, but its distribution is controlled and may be punishable when intended for human consumption or GHB synthesis.
- People’s Republic of China: GBL is regulated as a Class III drug precursor.
- Poland: GBL is classified as a drug, requiring licensing for various activities involving the substance.
- Russia: GBL is classified as a psychotropic substance with limited trafficking and strict penalties for unlicensed use.
- Sweden: GBL is classified as a health-endangering substance, with considerations for changes in its legal status.
- United Kingdom: GBL is legal for industrial purposes, but it is a controlled substance when intended for human ingestion.
- United States: GBL is regulated as a List I controlled chemical. It is also treated as a controlled substance under Schedule I of the Controlled Substances Act when intended for human consumption. Sales and distribution for industrial use are tightly regulated and require appropriate DEA licenses and compliance with strict security measures.
FAQ
1. What is GBL?
Gamma-Butyrolactone, commonly known as GBL, is a chemical compound that is used in various industrial and recreational applications. It is often used as a precursor to gamma-hydroxybutyric acid (GHB) and has a range of other uses.
2. How is GBL produced?
GBL is typically produced by the dehydrogenation of 1,4-butanediol at high temperatures in the presence of a copper catalyst. It can also be obtained through other chemical processes involving tetrahydrofuran (THF) or by transforming GABA via a diazonium intermediate.
3. What are the primary applications of GBL?
GBL serves as an intermediate chemical in the production of various other compounds, including polymers, solvents, and chemicals like methyl-2-pyrrolidone. It is also used in the synthesis of poly(4-hydroxybutyrate), an important polymer. In addition to its industrial uses, GBL has been misused as a recreational drug.
4. Is GBL safe for recreational use?
No, GBL is not safe for recreational use. It is a prodrug of GHB and can lead to dangerous health effects when misused. Overdosing on GBL can cause irrational behaviour, severe sickness, coma, and even death. Combining it with alcohol further increases the risks.
5. What are the legal considerations for GBL?
The legal status of GBL varies by country. In some places, it is classified as a controlled substance, while in others, it may be regulated as a precursor chemical. Possession and distribution laws differ depending on your location, so it’s essential to be aware of and comply with local regulations.
6. Can GBL be used as a nutritional supplement?
GBL has been marketed as a nutritional supplement due to its potential to increase growth hormone secretion. However, its use for this purpose has been banned by the FDA in some countries due to safety concerns.
7. Is GBL addictive?
Frequent and excessive use of GBL can lead to physical and psychological dependence. Users may experience withdrawal symptoms when attempting to quit or abstain from GBL use, especially if they have developed a tolerance.
8. What precautions should be taken when handling GBL?
When handling GBL, it’s essential to follow safety guidelines and take appropriate precautions, such as using protective gear and ensuring proper ventilation. Since it is a potentially hazardous chemical, it should be used in a controlled and regulated environment.
9. Can GBL be used in laboratory experiments?
Yes, GBL is sometimes used in laboratory experiments for various purposes, including the synthesis of certain chemicals. Researchers and scientists should follow safety protocols and work in compliance with relevant regulations when using GBL in a laboratory setting.
10. Is GBL used as a date rape drug?
Yes, GBL has been associated with cases of drug-facilitated sexual assault, and it is sometimes referred to as a date rape drug. This underscores the importance of being cautious and aware of your surroundings when in social settings where substances may be present.
References
1. Merck Index, 12th edition, 1632.
2. Lide, David R., ed. (2009-06-03). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, FL: CRC Press. ISBN 978-1-4200-9084-0. Archived from the original on 2011-07-16. Retrieved 2011-07-18.
3. “gamma-Butyrolactone”. www.chemsrc.com.
4. Anvisa (2023-03-31). “RDC Nº 784 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle” [Collegiate Board Resolution No. 784 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
5. Wolfgang Schwarz; Jürgen Schossig; Roland Rossbacher; Rolf Pinkos; Hartmut Höke (2019). “Butyrolactone”. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_495.pub2.
6. Schep, Leo J; Knudsen, Kai; Slaughter, Robin J; Vale, J Allister; Mégarbane, Bruno (2012). “The clinical toxicology of gamma-hydroxybutyrate, gamma-butyrolactone and 1,4-butanediol”. Clinical Toxicology. 50 (6): 458–470. doi:10.3109/15563650.2012.702218. ISSN 1556-3650.
7. Vose, J.; Tighe, T.; Schwartz, M.; Buel, E. (2001). “Detection of gamma-butyrolactone (GBL) as a natural component in wine”. Journal of Forensic Sciences. 46 (5): 1164–1167. doi:10.1520/JFS15116J. PMID 11569560.
8. Elliott, S.; Burgess, V. (2005). “The presence of gamma-hydroxybutyric acid (GHB) and gamma-butyrolactone (GBL) in alcoholic and non-alcoholic beverages”. Forensic Science International. 151 (2–3): 289–292. doi:10.1016/j.forsciint.2005.02.014. PMID 15939164.
9. “A Change to the Misuse of Drugs Act 1971: Control of GBL, 1,4-BD, BZP and related piperazine compounds, a further group of anabolic steroids and 2 non-steroidal agents, synthetic cannabinoid receptor agonists and oripavine” (PDF). Archived from the original (PDF) on 2014-10-06. Retrieved 2014-03-31.
10. Metsger, Leonid; Bittner, Shmuel (March 2000). “Autocatalytic Oxidation of Ethers with Sodium Bromate”. Tetrahedron. 56 (13): 1905–1910. doi:10.1016/S0040-4020(00)00098-3.
11. “Sandmeyer Reaction of GABA to GBL/GHB”. Retrieved 2018-06-14.
12. Micu, Alexandru (December 12, 2015). “New, fully recyclable and biodegradable plastic could change the world”. ZME Science. Retrieved 2015-12-13.
13. Hong, Miao; Chen, Eugene Y.-X. (2015). “Completely recyclable biopolymers with linear and cyclic topologies via ring-opening polymerization of γ-butyrolactone”. Nature Chemistry. 8 (1): 42–49. doi:10.1038/nchem.2391. PMID 26673263.
14. Hong, M.; Chen, E.Y.-X. (2016). “Towards Truly Sustainable Polymers: A Metal-Free Recyclable Polyester from Biorenewable Non-Strained γ-Butyrolactone”. Angewandte Chemie International Edition. 55 (13): 4188–4193. doi:10.1002/anie.201601092. PMID 26934184.
15. Wilson, Richard M. (December 1, 1996). “Space Electrochemical Research and Technology” (PDF) – via ntrs.nasa.gov.
16. [Tianqi Niu, Jing Lu, Rahim Munir, Jianbo Li, Dounya Barrit, Xu Zhang, Hanlin Hu, Zhou Yang, Aram Amassian, Kui Zhao, and Shengzhong (Frank) Liu: “Stable High-Performance Perovskite Solar Cells via Grain Boundary Passivation”, Advanced Materials, 2018, 1706576.]
17. Kobilinsky, Lawrence (2011-11-29). Forensic Chemistry Handbook. John Wiley & Sons. p. 386. ISBN 978-0-471-73954-8.
18. Teiber, J.F.; Draganov, D.I.; Du, B.N.L. (2003). “Lactonase and lactonizing activities of human serum paraoxonase (PON1) and rabbit serum PON3”. Biochemical Pharmacology. 66 (6): 887–896. doi:10.1016/S0006-2952(03)00401-5. PMID 12963475.
19. “Gamma-butyrolactone (GBL) Pre-Review Report” (PDF). 4 June 2012.
20. Van Cauter, E.; Plat, L.; Scharf, M. B.; Leproult, R.; Cespedes, S.; l’Hermite-Balériaux, M.; Copinschi, G. (1997). “Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young Men”. Journal of Clinical Investigation. 100 (3): 745–753. doi:10.1172/JCI119587. PMC 508244. PMID 9239423.
21. “Erowid GHB vault: FDA Warning about Gamma Butyrlactone”. Erowid. 1998-11-21. Retrieved 2013-10-10.
22. Meyer, Jerrold; Quenzer, Linda F. (2005). Psychopharmacology: Drugs, the Brain and Behavior. Sinauer. p. 370. ISBN 978-0-87893-534-5.
23. “USDOJ: U.S. Department of Justice Archive National Drug Intelligence Center” (PDF). Usdoj.gov. 2012-06-15. Retrieved 2014-01-22.
24. Galloway, G.P.; Frederick-Osborne, S.L.; Seymour, R.; Contini, S.E.; Smith, D.E. (2000). “Abuse and therapeutic potential of gamma-hydroxybutyric acid”. Alcohol. 20 (3): 263–269. doi:10.1016/S0741-8329(99)00090-7. PMID 10869868.
25. “‘There could be 100 comas in the year’: Paris police chief reacts to rise of GBL, GHB overdoses in clubs”. Resident Advisor. Archived from the original on 2018-04-20. Retrieved 2018-04-19.
26. “Drogue : ” L’interdiction de vente au public du GBL n’a rien changé à la consommation “”. Le Monde.fr (in French). 17 April 2018. Retrieved 2018-04-19.
27. van Nieuwenhuijzen, PS; McGregor, IS (1 August 2009). “Sedative and hypothermic effects of gamma-hydroxybutyrate (GHB) in rats alone and in combination with other drugs: assessment using biotelemetry”. Drug and Alcohol Dependence. 103 (3): 137–47. doi:10.1016/j.drugalcdep.2009.03.004. PMID 19446408.
28. Edwards, Richard (23 July 2009). “Coroner’s ‘Russian roulette’ warning over GBL party drug”. The Telegraph. Retrieved May 1, 2012.
29. “GBL/GHB”. London Friend. Retrieved 18 August 2014.
30. “GHB and GBL”. GMFA. Retrieved 18 August 2014.
31. Casciani, Dominic (23 December 2009). “GBL drug death identified by UK doctors”. BBC News. Retrieved May 1, 2012.
32. Karila, Laurent; Novarin, Johanne; Megarbane, Bruno; Cottencin, Olivier; Dally, Sylvain; Lowenstein, William; Reynaud, Michel (1 October 2009). “[Gamma-hydroxybutyric acid (GHB): more than a date rape drug, a potentially addictive drug]”. Presse Médicale. 38 (10): 1526–1538. doi:10.1016/j.lpm.2009.05.017. ISSN 2213-0276. PMID 19762202.
33. “GHB addiction, GHB physical n psychological dependency levels”. Archived from the original on July 26, 2010.
34. Santos C, Olmedo RE (2017). “Sedative-Hypnotic Drug Withdrawal Syndrome: Recognition And Treatment”. Emerg Med Pract. 19 (3): 1–20. PMID 28186869.
35. “Crew 2000 | GHB/ GBL Dependancy [sic] | | Drugs information, advice & support, Scotland, UK”. Archived from the original on 2016-03-19. Retrieved 2010-08-06.
36. “LAW AND JUSTICE LEGISLATION AMENDMENT (SERIOUS DRUG OFFENCES AND OTHER MEASURES) ACT 2005 NO. 129, 2005 – SCHEDULE 1 – Serious drug offences”.
37. “GHB – Alcohol and Drug Foundation”. adf.org.au. Retrieved 2023-07-17.
38. Branch, Legislative Services (May 19, 2023). “Consolidated federal laws of Canada, Controlled Drugs and Substances Act”. laws-lois.justice.gc.ca.
39. “section 7c of chapter B of part A of the 1st appendix of the Dangerous Drugs Act 1973”. www.nevo.co.il.
40. “wetten.nl – Regeling – Opiumwet – BWBR0001941”. Wetten.nl (in Dutch). 19 July 2019. Retrieved 19 July 2019.
41. “Webwinkels gestopt met handel in GBL”. Emerce (in Dutch). 9 December 2013. Retrieved 9 December 2013.
42. “国务院办公厅关于同意将α-苯乙酰乙酸甲酯等6种物质列入易制毒化学品品种目录的函” (in Simplified Chinese). The State Council – The People’s Republic of China. 7 June 2021. Retrieved 11 October 2021.
43. “Główny Inspektorat Farmaceutyczny”.
44. “Socialutskottets betänkande 2010/11:SoU5 – Riksdagen”.
45. “UK Statutory Instrument 2011 No. 448”. 2011-02-18.
46. “The Misuse of Drugs Act 1971 (Amendment) Order 2022”.
47. “Information Bulletin: GHB Analogs; GBL, BD, GHV, and GVL”. www.justice.gov.