Summary
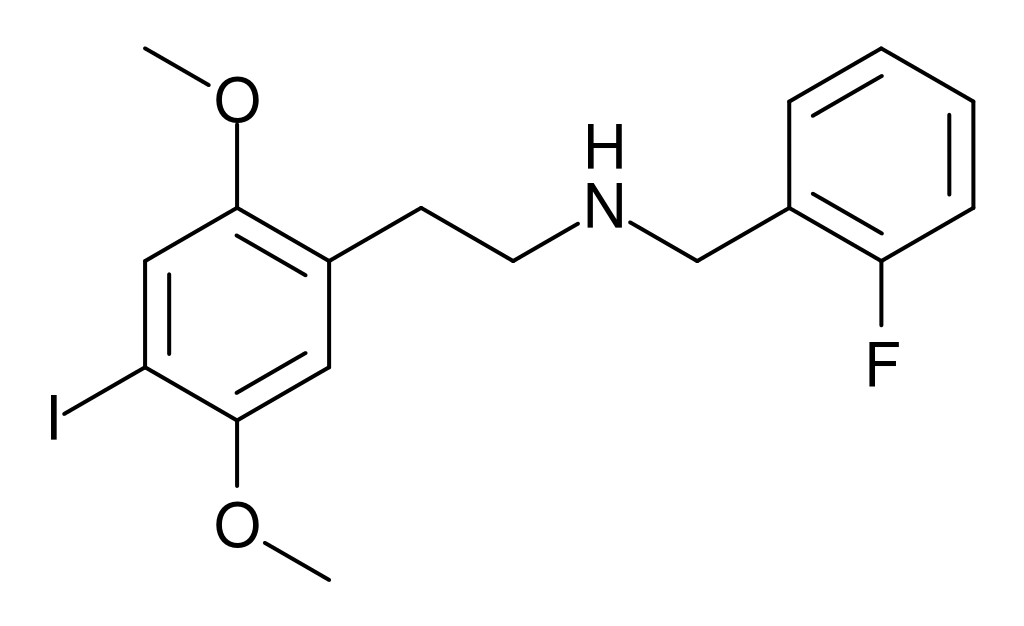
25I-NBF (2C-I-NBF, NBF-2C-I, Cimbi-21) is a compound derived from the phenethylamine hallucinogen 2C-I. It exhibits potent partial agonism for the human 5-HT2A receptor, known for its involvement in various neurological processes. Notably, it isn’t very objective towards the β-arrestin 2 coupled signalling pathway, indicating its complex interaction within the receptor system. In research, its 11C radiolabeled form has been explored as a potential ligand for mapping the distribution of 5-HT2A receptors in the brain, primarily utilizing positron emission tomography (PET).
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 919797-21-0 |
|---|---|
| PubChem CID | 57469209 |
| ChemSpider | 24751866 |
| UNII | MR8FRH5W3C |
| CompTox Dashboard (EPA) | DTXSID00726753 |
| Chemical and physical data | |
| Formula | C17H19FINO2 |
| Molar mass | 415.247 g·mol−1 |
Legality
In Hungary, Japan, Latvia, and Vermont, the possession and use of 25I-NBF are prohibited by law.
In Sweden, the Riksdag classified 25I-NBF under Swedish schedule I (“substances, plant materials, and fungi which normally do not have medical use”) as of January 26, 2016. The classification was published by the Medical Products Agency (MPA) in regulation HSLF-FS 2015:35, with the compound listed as 25I-NBF and 2-(4-jodo-2,5-dimetoxifenyl)-N-(2-fluorophenyl)etanamin.
Similarly, 25I-NBF is categorized as a Class A drug in the United Kingdom according to the N-benzyl phenethylamine catch-all clause in the Misuse of Drugs Act 1971.
FAQ
- What is 25I-NBF?
25I-NBF is a synthetic hallucinogenic compound and a derivative of the phenethylamine 2C-I. It is known for its psychedelic effects and is part of the NBOMe family of drugs. - How is 25I-NBF used?
25I-NBF is typically consumed orally, but it can also be taken sublingually or through other routes of administration. It is often found in the form of blotters, powders, or liquid solutions. - What are the effects of 25I-NBF?
The effects of 25I-NBF include visual and sensory distortions, changes in mood, altered perception of time, and intensified sensory experiences. Users may also experience shifts in thought patterns and heightened emotional states. - Are there any risks associated with 25I-NBF use?
Yes, 25I-NBF is known to have significant health risks. It can lead to adverse reactions such as increased heart rate, elevated blood pressure, and potential psychological distress. Overdosing on 25I-NBF can result in severe complications, including life-threatening situations. - Is 25I-NBF legal?
The legal status of 25I-NBF varies by country and region. It is often classified as a controlled substance due to its hallucinogenic properties and associated risks. Always check the specific regulations in your area. - Is 25I-NBF addictive?
There is limited research on the addictive potential of 25I-NBF. However, substances with hallucinogenic properties are generally not associated with physical dependence. Psychological dependence can still occur in some cases. - Are there any medical uses for 25I-NBF?
No, 25I-NBF is not approved for any medical purposes. It is primarily used for recreational or research purposes, but its use is strongly discouraged due to its potential dangers. - What should I do if I or someone I know experiences adverse effects from 25I-NBF?
If you or someone experiences negative effects or overdoses on 25I-NBF, seek immediate medical assistance. Call emergency services for help and follow their instructions. Do not delay in seeking professional medical care in such situations. - How can one minimize the risks associated with 25I-NBF use?
The best way to minimize the risks associated with 25I-NBF is to avoid its use entirely. Education about the potential dangers and harm reduction practices can also help individuals make informed decisions and take necessary precautions. - What are the long-term effects of 25I-NBF use?
Research on the long-term effects of 25I-NBF is limited. However, the potential risks of continued use may include persistent psychological distress, changes in cognitive function, and other adverse health outcomes. It is crucial to prioritize personal well-being and safety when considering substance use.
References
- Anvisa (2023-07-24). “RDC Nº 804 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial” [Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- Braden MR, Parrish JC, Naylor JC, Nichols DE (December 2006). “Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists”. Molecular Pharmacology. 70 (6): 1956–1964. doi:10.1124/mol.106.028720. PMID 17000863. S2CID 15840304.
- Braden MR (2007). Towards a biophysical understanding of hallucinogen action (Ph.D. thesis). Purdue University. ProQuest 304838368.
- Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL (March 2014). “Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists”. ACS Chemical Neuroscience. 5 (3): 243–249. doi:10.1021/cn400216u. PMC 3963123. PMID 24397362.
- Hansen M (2010-12-16). Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain (Ph.D. thesis). University of Copenhagen. doi:10.13140/RG.2.2.33671.14245.
- Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, et al. (April 2011). “Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers”. European Journal of Nuclear Medicine and Molecular Imaging. 38 (4): 681–693. doi:10.1007/s00259-010-1686-8. PMID 21174090. S2CID 12467684.
- “A Magyarországon megjelent, a Kábítószer és Kábítószer-függőség Európai Megfigyelő Központjának Korai Jelzőrendszerébe (EMCDDA EWS) 2005 óta bejelentett ellenőrzött anyagok büntetőjogi vonatkozású besorolása” [Criminal classification of controlled substances published in Hungary and notified to the European Monitoring Center for Drugs and Drug Addiction (EMCDDA EWS) since 2005] (PDF) (in Hungarian).
- “指定薬物名称・構造式一覧(平成27年9月16日現在)” (PDF) (in Japanese). 厚生労働省. 16 September 2015. Retrieved 8 October 2015.
- “Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām vielām un prekursoriem” [Regulations on Narcotic Drugs, Psychotropic Substances and Precursors Controlled in Latvia]. Ministry of Health of the Republic of Latvia (in Latvian). Archived from the original on 2016-03-04. Retrieved 2015-10-15.
- “Regulated Drugs Rule” (PDF). Vermont Department of Health. Archived from the original (PDF) on 5 June 2016. Retrieved 14 October 2015.
- Ödman P. “Gemensamma författningssamlingen avseende hälso- och sjukvård, socialtjänst, läkemedel, folkhälsa m.m.” [Joint constitutional collection on health care, social services, pharmaceuticals, public health, etc.] (PDF). Lakemedelsverket (in Swedish).
- “The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014”. www.legislation.gov.uk.
- “Explore N-(2C-I)-Fentanyl | PiHKAL · info”. isomerdesign.com.