Summary
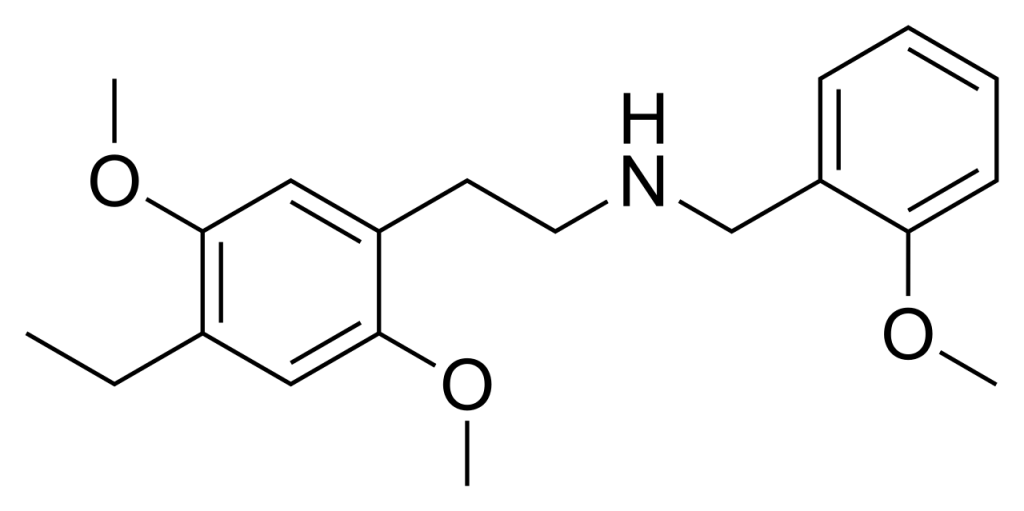
25E-NBOMe (also known as 2C-E-NBOMe or NBOMe-2C-E) is a chemical derivative originating from the phenethylamine 2C-E. It exhibits analogous behaviour to its counterparts, including 25I-NBOMe, known for their powerful activation of the 5HT2A receptor. This substance has been circulated as a recreational drug, eliciting effects comparable to related compounds like 25I-NBOMe and 25C-NBOMe in human subjects.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1354632-14-6 |
|---|---|
| PubChem CID | 118796522 |
| ChemSpider | 52085495 |
| UNII | 02HX6G35T3 |
| CompTox Dashboard (EPA) | DTXSID901014188 |
| Chemical and physical data | |
| Formula | C20H27NO3 |
| Molar mass | 329.440 g·mol−1 |
Toxicity
NBOMe substances are frequently linked to severe toxicity and fatalities. Scientific investigations into the NBOMe family of compounds have revealed their neurotoxic and cardiotoxic properties. Commonly, users of NBOMe encounter issues such as sympathomimetic toxicity, including vasoconstriction, hypertension, tachycardia, and hallucinations. Symptoms associated with NBOMe poisoning encompass agitation, aggression, seizures, hyperthermia, diaphoresis, hypertonia, rhabdomyolysis, and even death. Instances of NBOMe intoxication often exhibit signs resembling serotonin syndrome, with the likelihood of seizures being notably higher than other psychedelic substances.
The sale of NBOMe and NBOHs as LSD on blotter papers is not uncommon despite their bitter taste and distinct safety profiles. Unlike LSD, known for its low incidence of acute toxicity at recreational doses, fatalities associated with NBOMe intoxication suggest a significant number of users ingested the substance under the false belief that it was LSD. The lack of comprehensive documentation regarding NBOMe consumption leaves the long-term effects of the importance largely unknown. Typically, NBOMe compounds are administered sublingually, resulting in tongue and mouth numbness, followed by a metallic chemical taste, a critical differentiator from LSD.
Regarding its neurotoxic and cardiotoxic actions, many NBOMe compounds exhibit potent agonist activity at additional 5-HT receptors, with prolonged activation of 5-HT2B potentially leading to cardiac valvulopathy, especially with high doses and chronic use. The compounds’ high affinity for adrenergic α1 receptors contributes to their stimulant-like cardiovascular effects. In laboratory studies, 25C-NBOMe has shown cytotoxic effects on various neuronal cell lines and cardiomyocytes, with implications for MAPK/ERK cascade activation and inhibition of Akt/PKB signalling pathways. Furthermore, preliminary research suggests the toxicity 25C-NBOMe on the development, heart, and brain health in zebrafish, rats, and Artemia salina, warranting further investigation, particularly concerning potential harm to pregnant women and their fetuses.
Emergency treatment for NBOMe poisoning involves managing acute intoxication symptoms through symptomatic treatments, including benzodiazepines, antipsychotic drugs, and antiarrhythmic agents like beta blockers. Specific interventions are also employed to address rhabdomyolysis, which may lead to critical complications like metabolic acidosis and acute kidney injury.
Legality
In the United Kingdom, the categorization of this substance as a Class A drug stems from the inclusion of the N-benzylphenethylamine catch-all clause in the Misuse of Drugs Act 1971.
Similarly, in Sweden, the national public health agency officially classified 25E-NBOMe as a narcotic substance on January 18, 2019.
FAQ
- What is 25E-NBOMe?
- 25E-NBOMe, also known as 2C-E-NBOMe or NBOMe-2C-E, is a chemical derivative derived from the phenethylamine 2C-E. It is known for its potent effects on the 5HT2A receptor, similar to other compounds in the NBOMe family.
- What are the expected effects of 25E-NBOMe?
- 25E-NBOMe is reported to produce effects comparable to those of related compounds, such as 25I-NBOMe and 25C-NBOMe. These effects often include hallucinations and other psychoactive experiences.
- What are the risks associated with 25E-NBOMe use?
- 25E-NBOMe has been linked to potentially severe risks, including life-threatening toxicity. It is essential to be aware of the possible adverse reactions, including neurotoxic and cardiotoxic effects.
- How does 25E-NBOMe compare to other substances?
- 25E-NBOMe shares similarities with other NBOMe compounds regarding their mode of action and potential effects. Understanding these comparisons can help users and researchers better comprehend its potential risks and benefits.
- What are the legal implications of 25E-NBOMe use?
- The legal status of 25E-NBOMe varies from country to country. It is essential to stay informed about this substance’s legal framework to avoid any legal repercussions.
- What precautions should be taken when using 25E-NBOMe?
- Users should exercise caution when consuming 25E-NBOMe or any related substances. This includes being aware of the proper dosage, understanding potential interactions with other medications, and being conscious of the associated health risks.
- What are the long-term effects of 25E-NBOMe use?
- Research on the long-term effects of 25E-NBOMe is still limited. It is crucial to consider the potential long-term impacts on physical and mental health and stay informed about the latest scientific findings in this area.
- How can one seek help in case of 25E-NBOMe-related emergencies?
- In case of emergencies or adverse reactions related to 25E-NBOMe use, it is vital to seek immediate medical assistance. Understanding the appropriate steps for emergency intervention can help prevent serious health complications.
References
- Anvisa (2023-07-24). “RDC Nº 804 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial” [Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL (March 2014). “Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists”. ACS Chemical Neuroscience. 5 (3): 243–249. doi:10.1021/cn400216u. PMC 3963123. PMID 24397362.
- Hansen M (2010-12-16). Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain (Ph.D. thesis). University of Copenhagen. doi:10.13140/RG.2.2.33671.14245.
- Sean I, Joe R, Jennifer S, and Shaun G (28 March 2022). “A cluster of 25B-NBOH poisonings following exposure to powder sold as lysergic acid diethylamide (LSD)”. Clinical Toxicology. 60 (8): 966–969. doi:10.1080/15563650.2022.2053150. PMID 35343858. S2CID 247764056.
- Amy E, Katherine W, John R, Sonyoung K, Robert J, Aaron J (December 2018). “Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT2A receptors”. Biochemical Pharmacology. 158: 27–34. doi:10.1016/j.bcp.2018.09.024. PMC 6298744. PMID 30261175.
- Jolanta Z, Monika K, and Piotr A (26 February 2020). “NBOMes–Highly Potent and Toxic Alternatives of LSD”. Frontiers in Neuroscience. 14: 78. doi:10.3389/fnins.2020.00078. PMC 7054380. PMID 32174803.
- Lipow M, Kaleem SZ, Espiridion E (30 March 2022). “NBOMe Toxicity and Fatalities: A Review of the Literature”. Transformative Medicine. 1 (1): 12–18. doi:10.54299/tmed/msot8578. ISSN 2831-8978. S2CID 247888583.
- Micaela T, Sabrine B, Raffaella A, Giorgia C, Beatrice M, Tatiana B, Federica B, Giovanni S, Francesco B, Fabio G, Krystyna G, Matteo M (21 April 2022). “Effect of -NBOMe Compounds on Sensorimotor, Motor, and Prepulse Inhibition Responses in Mice in Comparison With the 2C Analogs and Lysergic Acid Diethylamide: From Preclinical Evidence to Forensic Implication in Driving Under the Influence of Drugs”. Front Psychiatry. 13: 875722. doi:10.3389/fpsyt.2022.875722. PMC 9069068. PMID 35530025.
- Cristina M, Matteo M, Nicholas P, Maria C, Micaela T, Raffaella A, Maria L (12 December 2019). “Neurochemical and Behavioral Profiling in Male and Female Rats of the Psychedelic Agent 25I-NBOMe”. Frontiers in Pharmacology. 10: 1406. doi:10.3389/fphar.2019.01406. PMC 6921684. PMID 31915427.
- Anna R, Dino L, Julia R, Daniele B, Marius H, Matthias L (December 2015). “Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs)”. Neuropharmacology. 99: 546–553. doi:10.1016/j.neuropharm.2015.08.034. ISSN 1873-7064. PMID 26318099. S2CID 10382311.
- David W, Roumen S, Andrew C, Paul D (6 February 2015). “Prevalence of use and acute toxicity associated with the use of NBOMe drugs”. Clinical Toxicology. 53 (2): 85–92. doi:10.3109/15563650.2015.1004179. PMID 25658166. S2CID 25752763.
- Humston C, Miketic R, Moon K, Ma P, Tobias J (2017-06-05). “Toxic Leukoencephalopathy in a Teenager Caused by the Recreational Ingestion of 25I-NBOMe: A Case Report and Review of Literature”. Journal of Medical Cases. 8 (6): 174–179. doi:10.14740/jmc2811w. ISSN 1923-4163.
- Justin P, Stephen R, Kylin A, Alphonse P, Michelle P (2015). “Analysis of 25I-NBOMe, 25B-NBOMe, 25C-NBOMe and Other Dimethoxyphenyl-N-[(2-Methoxyphenyl) Methyl]Ethanamine Derivatives on Blotter Paper”. Journal of Analytical Toxicology. 39 (8): 617–623. doi:10.1093/jat/bkv073. PMC 4570937. PMID 26378135.
- Morini L, Bernini M, Vezzoli S, Restori M, Moretti M, Crenna S, et al. (October 2017). “Death after 25C-NBOMe and 25H-NBOMe consumption”. Forensic Science International. 279: e1–e6. doi:10.1016/j.forsciint.2017.08.028. PMID 28893436.
- Byard RW, Cox M, Stockham P (November 2016). “Blunt Craniofacial Trauma as a Manifestation of Excited Delirium Caused by New Psychoactive Substances”. Journal of Forensic Sciences. 61 (6): 1546–1548. doi:10.1111/1556-4029.13212. PMID 27723094. S2CID 4734566.
- Sabastian LP, Christoffer B, Martin H, Martin AC, Jan K, Jesper LK (14 February 2014). “Correlating the Metabolic Stability of Psychedelic 5-HT2A Agonists with Anecdotal Reports of Human Oral Bioavailability”. Neurochemical Research. 39 (10): 2018–2023. doi:10.1007/s11064-014-1253-y. PMID 24519542. S2CID 254857910.
- Adam H (18 January 2017). “Pharmacology and Toxicology of N-Benzylphenethylamine (“NBOMe”) Hallucinogens”. Neuropharmacology of New Psychoactive Substances. Current Topics in Behavioral Neurosciences. Vol. 32. Springer. pp. 283–311. doi:10.1007/7854_2016_64. ISBN 978-3-319-52444-3. PMID 28097528.
- Boris D, Cristian C, Marcelo K, Edwar F, Bruce KC (August 2016). “Analysis of 25 C NBOMe in Seized Blotters by HPTLC and GC–MS”. Journal of Chromatographic Science. 54 (7): 1153–1158. doi:10.1093/chromsci/bmw095. PMC 4941995. PMID 27406128.
- Francesco SB, Ornella C, Gabriella A, Giuseppe V, Rita S, Flaminia BP, Eduardo C, Pierluigi S, Giovanni M, Guiseppe B, Fabrizio S (3 July 2014). “25C-NBOMe: preliminary data on pharmacology, psychoactive effects, and toxicity of a new potent and dangerous hallucinogenic drug”. BioMed Research International. 2014: 734749. doi:10.1155/2014/734749. PMC 4106087. PMID 25105138.
- Adam JP, Simon HT, Simon LH (September 2021). “Pharmacology and toxicology of N-Benzyl-phenylethylamines (25X-NBOMe) hallucinogens”. Novel Psychoactive Substances: Classification, Pharmacology and Toxicology (2 ed.). Academic Press. pp. 279–300. doi:10.1016/B978-0-12-818788-3.00008-5. ISBN 978-0-12-818788-3. S2CID 240583877.
- Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, Roth BL (Dec 2000). “Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications”. Circulation. 102 (23): 2836–41. doi:10.1161/01.CIR.102.23.2836. PMID 11104741.
- Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, Sun JH, Link JR, Abbaszade I, Hollis JM, Largent BL, Hartig PR, Hollis GF, Meunier PC, Robichaud AJ, Robertson DW (Jan 2000). “Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine”. Molecular Pharmacology. 57 (1): 75–81. PMID 10617681.
- Roth BL (Jan 2007). “Drugs and valvular heart disease”. The New England Journal of Medicine. 356 (1): 6–9. doi:10.1056/NEJMp068265. PMID 17202450.
- Xu P, Qiu Q, Li H, Yan S, Yang M, Naman CB, et al. (26 February 2019). “25C-NBOMe, a Novel Designer Psychedelic, Induces Neurotoxicity 50 Times More Potent Than Methamphetamine In Vitro”. Neurotoxicity Research. 35 (4): 993–998. doi:10.1007/s12640-019-0012-x. PMID 30806983. S2CID 255763701.
- Álvarez-Alarcón N, Osorio-Méndez JJ, Ayala-Fajardo A, Garzón-Méndez WF, Garavito-Aguilar ZV (2021). “Zebrafish and Artemia salina in vivo evaluation of the recreational 25C-NBOMe drug demonstrates its high toxicity”. Toxicology Reports. 8: 315–323. doi:10.1016/j.toxrep.2021.01.010. ISSN 2214-7500. PMC 7868744. PMID 33598409.
- “The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014”. www.legislation.gov.uk.
- “Sexton nya ämnen klassas som narkotika eller hälsofarlig vara” (in Swedish). Folkhälsomyndigheten. 18 January 2019.