Contents
Summary
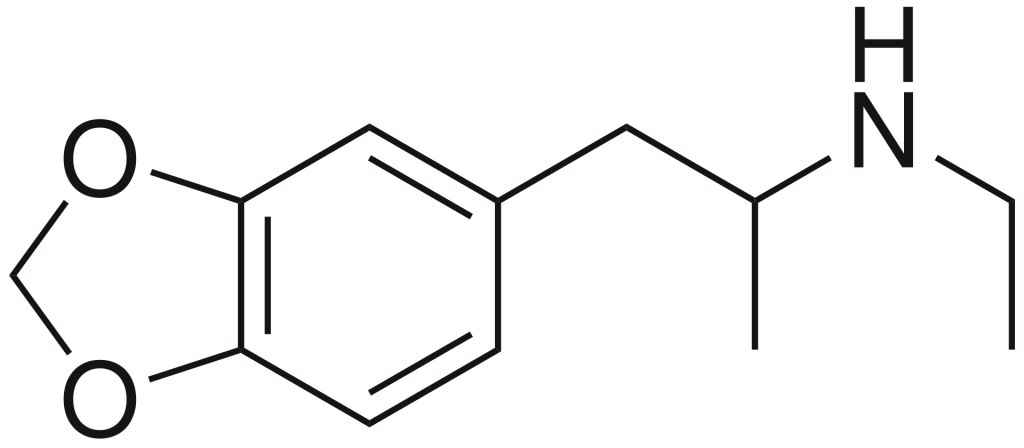
3,4-Methylenedioxy-N-ethylamphetamine (MDEA) is a psychoactive drug with empathogenic properties, sometimes referred to as MDE or colloquially known as Eve. It falls into the category of substituted amphetamines and substituted methylenedioxyphenethylamines. MDEA is a substance that releases and inhibits the reuptake of serotonin, norepinephrine, and dopamine in the brain.
It’s important to note that the possession of MDEA is prohibited in most countries. However, limited exceptions may exist for scientific and medical research purposes.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 82801-81-8 |
|---|---|
| PubChem CID | 105039 |
| ChemSpider | 94775 |
| UNII | ML1I4KK67B |
| ChEBI | CHEBI:132237 |
| CompTox Dashboard (EPA) | DTXSID70860971 |
| ECHA InfoCard | 100.231.031 |
| Chemical and physical data | |
| Formula | C12H17NO2 |
| Molar mass | 207.273 g·mol−1 |
Uses
Medical Use
As of now, MDEA does not have any established medical applications.
Recreational Use
MDEA is utilized recreationally like MDMA, commonly known as ecstasy. However, it’s worth noting that the subjective effects of MDEA are milder and of shorter duration. Alexander Shulgin also mentioned its potential to induce a stoning effect at high doses.
MDEA is most commonly consumed orally, with recreational doses typically reaching 100 to 200 mg. Occasionally, MDEA may be found as an adulterant in ecstasy pills. Studies from the 1990s revealed that MDEA was present in approximately four percent of ecstasy tablets.
Adverse effects
The documented adverse effects associated with MDEA use encompass the following:
- Hyperthermia
- Mydriasis (dilated pupils)
- Loss of appetite
Overdose
Reported symptoms of overdose related to MDEA usage encompass the following:
- Disseminated intravascular coagulation
- Muscle rigidity
- Rhabdomyolysis
- Convulsions
- Tachycardia
- Hypotension
- Sweating
History, society, and culture
Alexander Shulgin embarked on research involving methylenedioxy compounds during the 1960s. In a lab notebook entry dated 1967, Shulgin briefly noted that a colleague reported experiencing no discernible effects from a 100 mg dose of the substance. Shulgin later provided a detailed characterization of the essence in his book PiHKAL.
In the United States, MDEA was introduced for recreational use in 1985 as a legal alternative following the recent ban on MDMA. However, MDEA was classified as a Schedule 1 substance in the United States on August 13, 1987, by the Federal Analog Act.
FAQ
1. What is 3,4-methylenedioxy-N-ethylamphetamine (MDEA)?
3,4-Methylenedioxy-N-ethylamphetamine, often referred to as MDEA or colloquially known as Eve, is a psychoactive substance with empathogenic properties. It falls under the categories of substituted amphetamines and substituted methylenedioxyphenethylamines.
2. Does MDEA have any accepted medical uses?
Currently, MDEA does not have any recognized medical applications.
3. How is MDEA used recreationally, and what are its effects compared to MDMA (ecstasy)?
MDEA is commonly used recreationally, like MDMA (ecstasy). However, the subjective effects of MDEA are reported to be milder and shorter-lasting than those of MDMA. Users may experience enhanced empathy and emotional closeness.
4. Are there recommended recreational doses for MDEA?
When taken orally, recreational doses of MDEA typically range from 100 to 200 mg. It’s important to note that individual responses to the substance may vary.
5. What are the reported adverse effects of MDEA use?
Reported adverse effects of MDEA use include hyperthermia (elevated body temperature), mydriasis (dilated pupils), and loss of appetite.
6. What are the symptoms of an MDEA overdose?
In cases of MDEA overdose, symptoms may include disseminated intravascular coagulation, muscle rigidity, rhabdomyolysis, convulsions, tachycardia (rapid heart rate), hypotension (low blood pressure), and profuse sweating.
7. Who researched MDEA, and when was it introduced recreationally in the United States?
Alexander Shulgin researched MDEA in the 1960s and later documented it in his book PiHKAL. In the United States, MDEA was introduced for recreational use in 1985 as a legal substitute for MDMA. However, it was classified as a Schedule 1 substance under the Federal Analog Act on August 13, 1987.
8. Is MDEA legal?
The legal status of MDEA varies by country and region. In many places, it is classified as a controlled or illegal substance. Check local drug laws and regulations before considering the use or possession of MDEA.
9. Is MDEA addictive?
MDEA may have the potential for psychological dependence when used regularly and excessively. However, there is limited research on its addiction potential compared to other substances.
10. Where can I find more information about MDEA and its legal status?
For additional information about MDEA, its legal status in your area, and resources related to its use, consult local drug enforcement agencies, healthcare professionals, or addiction support services. Staying informed about drug laws and regulations is crucial for making responsible decisions.
References
- Freudenmann RW, Spitzer M (2004). “The Neuropsychopharmacology and Toxicology of 3,4-methylenedioxy-N-ethyl-amphetamine (MDEA)”. CNS Drug Reviews. 10 (2): 89–116. This comprehensive review explores the neuropsychopharmacology and toxicology of MDEA, providing valuable insights into its effects and potential risks.
- Anvisa (2023-07-24). “RDC Nº 804 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial” [Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). This official document outlines the regulatory control of substances like MDEA in Brazil, shedding light on its legal status.
- Tehan B, Hardern R, Bodenham A (June 1993). “Hyperthermia associated with 3,4-methylenedioxyethamphetamine (‘Eve’)”. Anaesthesia. 48 (6): 507–10. This research article discusses hyperthermia linked to substances like MDEA, providing insights into its potential adverse effects.
- Shulgin A. “#106 MDE: MDEA; EVE; N-Ethyl-MDA; 3,4-Methylenedioxy-N-ethylamphetamine”. Isomer Design. Alexander Shulgin’s work and descriptions of MDEA can be found in this resource, offering valuable historical context.
- Benzenhöfer U, Passie T (August 2010). “Rediscovering MDMA (ecstasy): the role of the American chemist Alexander T. Shulgin”. Addiction. 105 (8): 1355–61. This article delves into the contributions of Alexander Shulgin, including his involvement with substances like MDEA, offering a perspective on the rediscovery of MDMA.