Contents
Summary
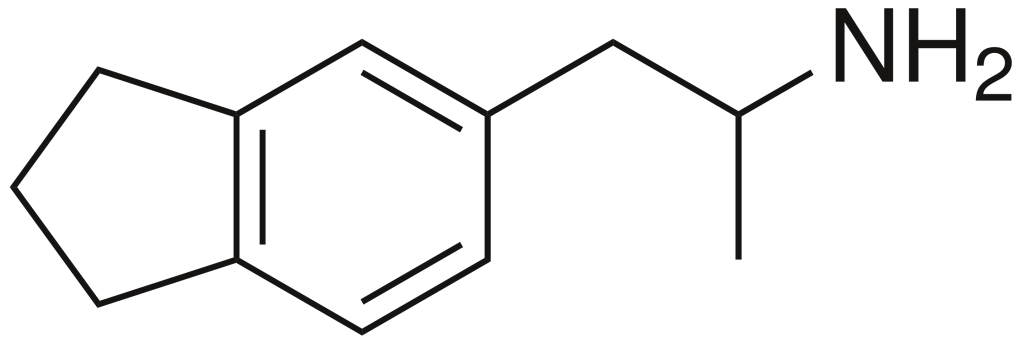
5-(2-Aminopropyl)-2,3-dihydro-1H-indene (commonly known as 5-APDI or indanylaminopropane/IAP) is a member of the amphetamine family, with diverse aliases including IAP (psychedelic), 2-API (2-aminopropylindane), indanametamine, and, occasionally, indanylamphetamine. This compound is recognized for its entactogenic and psychedelic properties.
5-APDI and Its Availability:
5-APDI has been accessible through online vendors on the internet and has been present as a designer drug since 2003. However, its popularity and availability have waned in recent years.
Pharmacological Properties:
In terms of its pharmacology, 5-APDI functions as a powerful, albeit weakly selective serotonin-releasing agent (SSRA). Its inhibitory concentrations (IC50 values) are approximately 82 nM for serotonin, 1,848 nM for dopamine, and 849 nM for norepinephrine reuptake.
Effects on Trained Animals:
When studied in trained animals, 5-APDI exhibits complete substitution for MBDB but not for amphetamine, although it does disrupt the effects of the latter at higher doses.
Legal Status:
5-APDI was classified as a Class B drug under the Misuse of Drugs Act 1971 as of June 10, 2014. This categorization restricts its possession, distribution, and use in the United Kingdom.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 13396-94-6 |
|---|---|
| PubChem CID | 192600 |
| ChemSpider | 167142 |
| UNII | 4PH68L63R9 |
| ChEMBL | ChEMBL6842 |
| CompTox Dashboard (EPA) | DTXSID00896945 |
| Chemical and physical data | |
| Formula | C12H17N |
| Molar mass | 175.275 g·mol−1 |
FAQ
1. What is 5-APDI?
5-APDI, or 5-(2-Aminopropyl)-2,3-dihydro-1H-indene, is a chemical compound classified under the amphetamine family. It is known for its entactogenic and psychedelic effects.
2. What are the other names for 5-APDI?
5-APDI is referred to by various aliases, including indanylaminopropane (IAP), IAP (psychedelic), 2-API (2-aminopropylindane), and indanametamine. On occasion, it may be incorrectly labeled as indanylamphetamine.
3. When did 5-APDI become available as a designer drug?
5-APDI has been encountered as a designer drug since 2003. It was made accessible through online vendors via the internet.
4. What are the effects of 5-APDI?
5-APDI is recognized for its entactogenic and psychedelic properties. These effects can vary among individuals and depend on factors such as dosage and individual tolerance.
5. Is 5-APDI safe to use?
The safety of 5-APDI is not well-documented due to limited research. Using substances with unclear safety profiles can be risky, and its use is generally discouraged.
6. How does 5-APDI impact serotonin, dopamine, and norepinephrine reuptake?
5-APDI functions as a potent but weakly selective serotonin-releasing agent (SSRA). It exhibits inhibitory concentrations (IC50 values) of approximately 82 nM for serotonin, 1,848 nM for dopamine, and 849 nM for norepinephrine reuptake.
7. What is the legal status of 5-APDI?
5-APDI was classified as a Class B drug under the Misuse of Drugs Act 1971 in the United Kingdom on June 10, 2014. This means it is illegal to possess, distribute, or use in the UK.
8. How can I find more information about 5-APDI?
For reliable information on 5-APDI and similar compounds, it is advisable to consult reputable sources, scientific literature, government health agencies, and harm reduction organizations. Staying informed is crucial when dealing with substances like 5-APDI.
9. Are there harm reduction strategies for using substances like 5-APDI safely?
Harm reduction strategies are essential when dealing with psychoactive substances. Prioritize safety, responsible use, and informed decision-making. Seek guidance from harm reduction organizations or healthcare professionals for advice on minimizing risks when using substances like 5-APDI.
10. What should I do if I suspect someone is using 5-APDI or a similar designer drug?
If you suspect someone is using designer drugs or facing substance abuse issues, encourage them to seek professional help and support. Reach out to healthcare providers, counselors, or addiction specialists for guidance and assistance.
References
- Casale JF, McKibben TD, Bozenko JS, Hays PA (2005). “Characterization of the “Indanylamphetamines””. Microgram Journal. 3 (1–2): 3–10. Archived from the original on 2009-03-17. Retrieved 2009-08-06.In 2005, a comprehensive study by Casale and colleagues delved into the characterization of the “Indanylamphetamines.” This research explored the properties of these compounds and their implications.
- Monte AP, Marona-Lewicka D, Cozzi NV, Nichols DE (November 1993). “Synthesis and pharmacological examination of benzofuran, indan, and tetralin analogues of 3,4-(methylenedioxy)amphetamine”. Journal of Medicinal Chemistry. 36 (23): 3700–6. doi:10.1021/jm00075a027. PMID 8246240.Back in November 1993, Monte, Marona-Lewicka, Cozzi, and Nichols conducted a pivotal study focusing on the synthesis and pharmacological evaluation of benzofuran, indan, and tetralin analogues of 3,4-(methylenedioxy)amphetamine. This research contributed valuable insights into related compounds.
- Parker MA, Marona-Lewicka D, Kurrasch D, Shulgin AT, Nichols DE (March 1998). “Synthesis and pharmacological evaluation of ring-methylated derivatives of 3,4-(methylenedioxy)amphetamine (MDA)”. Journal of Medicinal Chemistry. 41 (6): 1001–5. CiteSeerX 10.1.1.688.9559. doi:10.1021/jm9705925. PMID 9526575.In March 1998, Parker, Marona-Lewicka, Kurrasch, Shulgin, and Nichols embarked on a study involving the synthesis and pharmacological assessment of ring-methylated derivatives of 3,4-(methylenedioxy)amphetamine (MDA). This research further expanded our understanding of related chemical compounds and their effects.