Summary
5-MeO-DPT, alternatively referred to as 5-methoxy-N,N-Dipropyltryptamine, is a designer drug with psychedelic and entheogenic properties.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 69496-75-9 |
|---|---|
| PubChem CID | 14011047 |
| ChemSpider | 14106484 |
| UNII | AYW60P516B |
| ChEMBL | ChEMBL169328 |
| CompTox Dashboard (EPA) | DTXSID70219723 |
| Chemical and physical data | |
| Formula | C17H26N2O |
| Molar mass | 274.408 g·mol−1 |
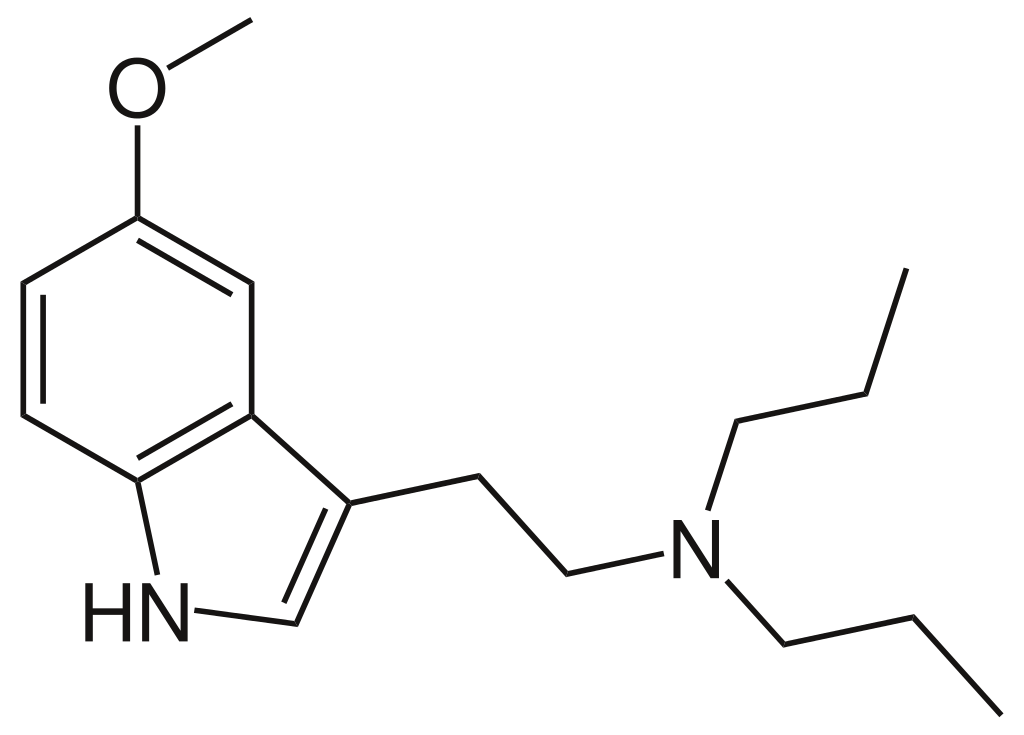
Chemistry
Its complete chemical nomenclature is N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-N-propylpropan-1-amine, belonging to the category of tryptamine derivatives.
Effects
There is limited knowledge regarding the subjective effects of 5-MeO-DPT, but its characteristics are likely akin to other psychedelic tryptamines/indoles such as 5-MeO-DiPT, 5-MeO-DMT, or DPT. However, it’s important to note that the durations of these mentioned substances can differ significantly.
Dosage
5-MeO-DPT is taken orally and is typically fully effective at dosages ranging from 3 to 10 mg. Its effects typically commence within three hours and generally persist for around 4 hours.
Legality
In the United States, 5-MeO-DPT is classified as a Schedule I controlled substance due to its status as a positional isomer of 5-Methoxy-N,N-diisopropyltryptamine (5-MeO-DiPT).
FAQ
- What is 5-MeO-DPT?
- 5-MeO-DPT, also known as 5-methoxy-N, N-Dipropyltryptamine, is a designer drug classified as a psychedelic and entheogenic substance. It is a derivative of the tryptamine class.
- What is the full chemical name of 5-MeO-DPT?
- The complete chemical name is N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-N-propylpropan-1-amine.
- How does 5-MeO-DPT compare to other psychedelic tryptamines?
- Although limited information is available about the subjective effects of 5-MeO-DPT, it is likely comparable to other psychedelic tryptamines, such as 5-MeO-DiPT, 5-MeO-DMT, or DPT, which are also classified as psychedelic tryptamines/indoles.
- What is the typical dosage and duration of 5-MeO-DPT effects?
- In most cases, a fully effective dosage of 5-MeO-DPT falls within the range of 3-10 mg. Effects usually manifest within three hours and can last around four hours.
- Is 5-MeO-DPT legal in the United States?
- No, 5-MeO-DPT is considered a Schedule I controlled substance in the United States. It falls under this classification due to its similarity to 5-Methoxy-N, N-diisopropyltryptamine (5-MeO-DiPT).
References
- Schulze-Alexandru M, Kovar KA, Vedani A (December 1999). “Quasi-atomistic Receptor Surrogates for the 5-HT2A Receptor: A 3D-QSAR Study on Hallucinogenic Substances”. Quantitative Structure-Activity Relationships. 18 (6): 548–560. CiteSeerX 10.1.1.669.1877. doi:10.1002/(SICI)1521-3838(199912)18:6<548::AID-QSAR548>3.0.CO;2-B.
- Gatch MB, Forster MJ, Janowsky A, Eshleman AJ (July 2011). “Abuse liability profile of three substituted tryptamines”. The Journal of Pharmacology and Experimental Therapeutics. 338 (1): 280–9. doi:10.1124/jpet.111.179705. PMC 3126641. PMID 21474568.
- Glennon RA, Gessner PK (April 1979). “Serotonin receptor binding affinities of tryptamine analogues”. Journal of Medicinal Chemistry. 22 (4): 428–32. doi:10.1021/jm00190a014. PMID 430481.
- Halberstadt AL, Geyer MA (September 2011). “Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens”. Neuropharmacology. 61 (3): 364–81. doi:10.1016/j.neuropharm.2011.01.017. PMC 3110631. PMID 21256140.
- Gessner PK, Godse DD, Krull AH, McMullan JM (March 1968). “Structure-activity relationships among 5-methoxy-n,n-dimethyltryptamine, 4-hydroxy-n,n-dimethyltryptamine (psilocin) and other substituted tryptamines”. Life Sciences. 7 (5): 267–77. doi:10.1016/0024-3205(68)90200-2. PMID 5641719.
- Lyon RA, Titeler M, Seggel MR, Glennon RA (January 1988). “Indolealkylamine analogs share 5-HT2 binding characteristics with phenylalkylamine hallucinogens”. European Journal of Pharmacology. 145 (3): 291–7. doi:10.1007/s00213-014-3557-7. PMC 4194234. PMID 24800892.
- Blough BE, Landavazo A, Decker AM, Partilla JS, Baumann MH, Rothman RB (October 2014). “Interaction of psychoactive tryptamines with biogenic amine transporters and serotonin receptor subtypes”. Psychopharmacology. 231 (21): 4135–44. doi:10.1007/s00213-014-3557-7. PMC 4194234. PMID 24800892.
- Glennon RA, Young R, Rosecrans JA, Kallman MJ (1980). “Hallucinogenic agents as discriminative stimuli: a correlation with serotonin receptor affinities”. Psychopharmacology. 68 (2): 155–8. doi:10.1007/BF00432133. PMID 6776558. S2CID 1674481.
- Nakamoto A, Namera A, Nishida M, Yashiki M, Kuramoto T, Kimura K (June 2007). “Identification and quantitative determination of 5-methoxy-N, N-di-n-propyltryptamine in urine by isotope dilution gas chromatography-mass spectrometry”. Forensic Toxicology. 25 (1): 1–7. doi:10.1007/s11419-006-0018-y. S2CID 9906203.
- Nakazono Y, Tsujikawa K, Kuwayama K, Kanamori T, Iwata YT, Miyamoto K, Kasuya F, Inoue H (January 2014). “Simultaneous determination of tryptamine analogues in designer drugs using gas chromatography–mass spectrometry and liquid chromatography–tandem mass spectrometry”. Forensic Toxicology. 32 (1): 154–61. doi:10.1007/s11419-013-0208-3. S2CID 25134125.
- Pham DN, Chadeayne AR, Golen JA, Manke DR (May 2021). “5-Meth-oxy-N,N-di-n-propyl-tryptamine (5-MeO-DPT): freebase and fumarate”. Acta Crystallographica Section E. 77 (Pt 5): 522–526. doi:10.1107/S2056989021003753. PMC 8100262. PMID 34026257.
- “Lists of: Scheduling Actions Controlled Substances Regulated Chemicals” (PDF). U.S. Department of Justice. February 2023. Retrieved 5 March 2023.