Contents
Summary
5-MeO-MiPT is a substance known for its psychedelic and hallucinogenic effects, occasionally employed by individuals as an entheogen. This compound shares both structural and pharmacodynamic similarities with other drugs like 5-MeO-DiPT, DiPT, and MiPT. Due to its strikingly comparable structure and effects, it is frequently utilized as an alternative to 5-MeO-DiPT.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 96096-55-8 |
|---|---|
| PubChem CID | 2763156 |
| ChemSpider | 2043845 |
| UNII | L0P1807EUY |
| ChEMBL | ChEMBL172139 |
| CompTox Dashboard (EPA) | DTXSID90242114 |
| ECHA InfoCard | 100.223.426 |
| Chemical and physical data | |
| Formula | C15H22N2O |
| Molar mass | 246.354 g·mol−1 |
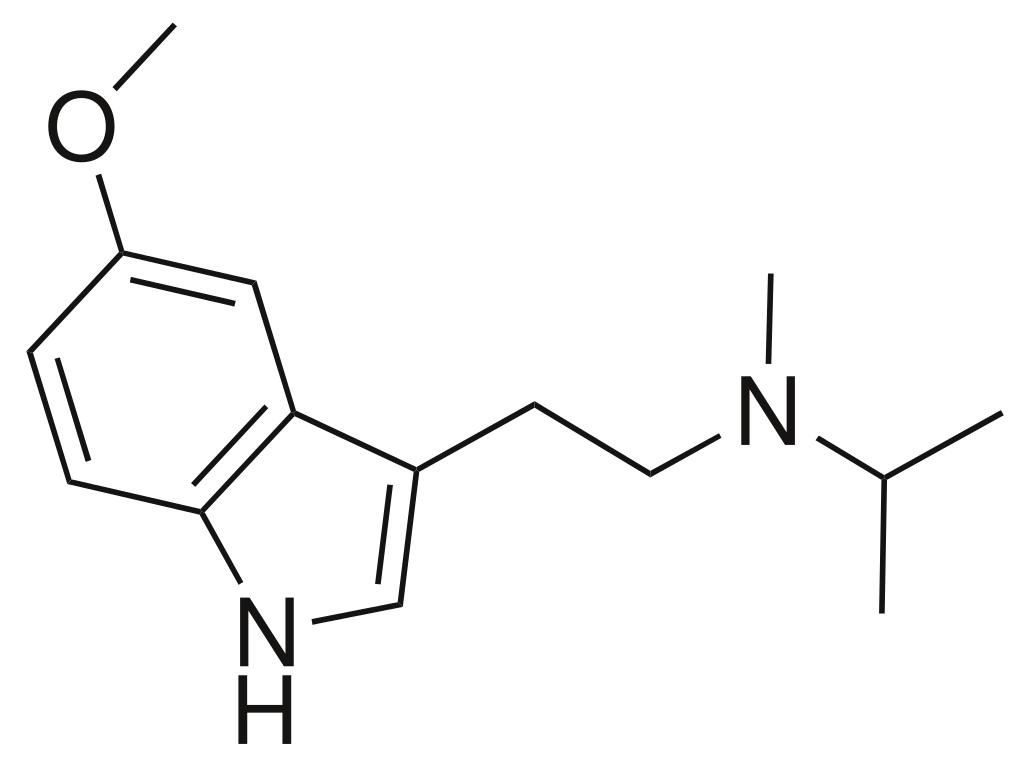
Chemistry
5-MeO-MiPT belongs to the category of compounds commonly referred to as tryptamines and represents the N-methyl-N-isopropyl counterpart of the psychedelic substance 5-MeO-DMT. Its complete chemical name is 5-methoxy-N-methyl-N-isopropyltryptamine.
When subjected to chemical tests, 5-MeO-MiPT demonstrates distinct reactions. Specifically, it induces a colour change in the Ehrlich reagent from purple, eventually fading to a faint blue. With the marquis reagent, the colour transition spans from yellow to black.
Effects
This compound is a close relative of the well-known drug 5-MeO-DiPT, affectionately nicknamed “foxy methoxy,” and it also goes by the moniker “moxy.” Some users have described experiencing the tactile sensations associated with 5-MeO-DiPT but without encountering certain undesirable side effects. When administered at higher doses, it can take on significantly more psychedelic qualities, occasionally drawing comparisons to 5-MeO-DMT. However, at doses ranging from 4 to 10 milligrams, users often find that 5-MeO-MiPT offers a highly euphoric and tactile experience. These effects can be notably stimulating, causing a substantial increase in heart rate. Additionally, it has the potential to heighten auditory perception to the extent that synesthetic phenomena, such as “feeling” or “tasting” sounds, may manifest.
Pharmacodynamics
| Binding Sites | Binding Affinity Ki (μM)[6] |
|---|---|
| 5-HT1A | 0.058 |
| 5-HT2A | 0.163 |
| 5-HT2C | 1.3 |
| D1 | >25 |
| D2 | >25 |
| D3 | >25 |
| α1A | >12 |
| α2A | 5.3 |
| TAAR1 | >15 |
| H1 | 3.9 |
| SERT | 3.3 |
| DAT | >26 |
| NET | >22 |
Dosage
| Smoked | Oral | |
|---|---|---|
| Threshold | 5 mg | 3 mg |
| Light | 5 – 10 mg | 3 – 7 mg |
| Common | 10 – 15 mg | 7 – 15 mg |
| Strong | 15 – 20 mg | 15 – 20 mg |
| Heavy | 20 mg + | 20 mg + |
Pharmacology
The hallucinogenic and entheogenic effects of 5-MeO-MiPT are primarily believed to stem from its interaction with the 5-HT2A receptor, acting as an agonist. However, additional mechanisms of action, including the potential inhibition of MAO, might also play a role.
While 5-MeO-MiPT exhibits the highest binding affinity for 5-HT1A receptors, it also demonstrates relatively strong binding to the serotonin and norepinephrine transporters (SERT and NET). Consequently, it functions as a moderately potent serotonin-norepinephrine reuptake inhibitor.
These diverse mechanisms may help clarify why numerous anecdotal accounts suggest that even modest doses of this compound have antidepressant and anxiolytic effects. This phenomenon finds parallels in conventional pharmacological approaches, as substances like venlafaxine, which acts as a serotonin-norepinephrine reuptake inhibitor (SNRI), are commonly prescribed for depression. Similarly, buspirone, a 5-HT1A agonist, is typically prescribed to alleviate anxiety.
Legal status
Canada
5-MeO-MiPT remains unscheduled in Canada.[citation needed]
China
As of October 2015, China classified 5-MeO-MiPT as a controlled substance.
Finland
In Finland, 5-MeO-MiPT is listed in a government decree prohibiting its sale on the consumer market.
Luxembourg
5-MeO-MiPT is not included in Luxembourg’s list of prohibited substances, making it a legal substance in the country.[14]
United Kingdom
In the United Kingdom, 5-MeO-MiPT is categorized as a Class A drug, along with most ring-hydroxy tryptamine ethers.[citation needed]
United States
At the federal level in the United States, 5-MeO-MiPT is not scheduled; however, it might be considered an analog of 5-MeO-DiPT. In such cases, its purchase, sale, or possession with intent to consume could be subject to prosecution under the Federal Analog Act.
Florida
“5-Methoxy-N-methyl-N-isopropyltryptamine” is designated as a Schedule I controlled substance in the state of Florida, rendering it illegal to buy, sell, or possess within the state.
FAQ
1. What is 5-MeO-MiPT?
5-MeO-MiPT, or 5-Methoxy-N-methyl-N-isopropyltryptamine, is a psychoactive compound that belongs to the tryptamine family. It is known for its psychedelic and hallucinogenic effects.
2. Is 5-MeO-MiPT legal in my country?
The legal status of 5-MeO-MiPT varies by country and region. It is important to check the specific drug laws and regulations in your area to determine their legality.
3. What are the effects of 5-MeO-MiPT?
5-MeO-MiPT can produce a range of effects, including psychedelic experiences, altered perception, and tactile sensations. These effects may vary depending on the dosage and individual reactions.
4. Is 5-MeO-MiPT safe to use?
The safety of 5-MeO-MiPT has not been extensively studied, and its effects can vary from person to person. Using any psychoactive substance carries inherent risks, and it’s important to exercise caution, be well-informed, and consider the potential consequences.
5. Can 5-MeO-MiPT be detected in drug tests?
5-MeO-MiPT may not be commonly included in standard drug tests, but some specialized tests might detect its presence. Always be aware of the specific substances included in any drug test you may be subject to.
6. What are the potential therapeutic uses of 5-MeO-MiPT?
The therapeutic potential of 5-MeO-MiPT has not been extensively researched. While some individuals and researchers explore the therapeutic use of psychedelics, it’s important to emphasize that these substances should only be used under the guidance of qualified medical professionals and in clinical settings.
7. Can I use 5-MeO-MiPT to self-treat mental health conditions?
Self-administering 5-MeO-MiPT for mental health conditions is not recommended. If you are seeking treatment for mental health issues, it is advisable to consult with a healthcare professional who can guide appropriate and evidence-based treatments.
8. Where can I find more information about 5-MeO-MiPT?
For further information on 5-MeO-MiPT, you can consult scientific literature or academic research or speak with a healthcare professional or substance abuse counsellor. Always prioritize your safety and well-being when considering the use of any psychoactive substance.
References
- Brazilian Regulation: Anvisa (2023-07-24) issued “Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control” (in Brazilian Portuguese). This regulation, dated 2023-07-24, was published in the Diário Oficial da União on 2023-07-25 and archived from the original on 2023-08-27, with retrieval on 2023-08-27.
- Research Insights: In 2004, Spratley T published “Analytical Profiles for Five ‘Designer’ Tryptamines” (PDF) in the Microgram Journal (Volume 3, Issue 1–2), which can be accessed and retrieved on 2013-10-09.
- DEA Proposal: On 2022-01-25, Carpenter DE reported in Lucid News that the DEA proposed adding five psychedelic compounds to Schedule 1. This information was retrieved on 2022-01-26.
- Qualitative Analysis: In January 2020, Palamar JJ and Acosta P conducted a qualitative descriptive analysis of the effects of psychedelic phenethylamines and tryptamines. This study was published in Human Psychopharmacology (Volume 35, Issue 1), with the digital object identifier (DOI) doi:10.1002/hup.2719, and is available through PMC 6995261. The PMID is 31909513.
- Educational Resource: The Drug Classroom provides information on “5-MeO-MiPT” and can be accessed at the website. The retrieval date for this resource is 2022-11-16.
- Receptor Interaction Study: Rickli A, Moning OD, Hoener MC, and Liechti ME published “Receptor Interaction Profiles of Novel Psychoactive Tryptamines Compared with Classic Hallucinogens” (PDF) in the European Neuropsychopharmacology (Volume 26, Issue 8) in August 2016. The DOI for this study is doi:10.1016/j.euroneuro.2016.05.001, and the PMID is 27216487. S2CID is 6685927.
- Psychedelic Compilation: “#40 5-MEO-MIPT” is featured in “TIHKAL,” an online resource available on Erowid, with retrieval on 2021-06-01.
- Comprehensive Information: Erowid Exp: Main Index provides detailed information on “5-MeO-MIPT (also 5-Methoxy-N,N-Methylisopropyltryptamine).” The retrieval date for this resource is 2021-06-01.
- Structural Variations: Repke DB, Grotjahn DB, and Shulgin AT explored “Psychotomimetic N-methyl-N-isopropyltryptamines” and their effects based on variations in aromatic oxygen substituents. This research was published in the Journal of Medicinal Chemistry (Volume 28, Issue 7) in July 1985. The DOI for this study is doi:10.1021/jm00145a007, and the PMID is 4009612.
- Neurotransmission Effects: Nagai F, Nonaka R, and Satoh Hisashi Kamimura K investigated “The Effects of Non-Medically Used Psychoactive Drugs on Monoamine Neurotransmission in Rat Brain.” This study was published in the European Journal of Pharmacology (Volume 559, Issue 2–3) in March 2007. The DOI is doi:10.1016/j.ejphar.2006.11.075, and the PMID is 17223101.
- Receptorome Study: Ray TS examined “Psychedelics and the Human Receptorome” in February 2010. This research was published in PLOS ONE (Volume 5, Issue 2) with the article’s DOI being doi:10.1371/journal.pone.0009019. The study is available through PMC 2814854, and the PMID is 20126400.
- Chinese Classification: In China, the China Food and Drug Administration released a notice on September 27, 2015, titled “Notice on Printing and Distributing the Measures for the Scheduling of Non-Pharmaceutical Narcotic Drugs and Psychotropic Substances” (in Chinese). This information was archived from the original on 2015-10-01, with retrieval on 2015-10-01.
- Finnish Regulation: The Finnish regulation “Valtioneuvoston asetus kuluttajamarkkinoilta… 1130/2014” is available at the FINLEX® website, with retrieval on 2023-07-11.
- Luxembourg Legal Framework: Luxembourg’s “Law of February 19, 1973 concerning the sale of medicinal substances and the fight against drug addiction” is detailed in the Journal officiel du Grand-Duché de Luxembourg (Official Journal of the Grand Duchy of Luxembourg) (in French).
- U.S. Controlled Substances: The United States’ “