Summary
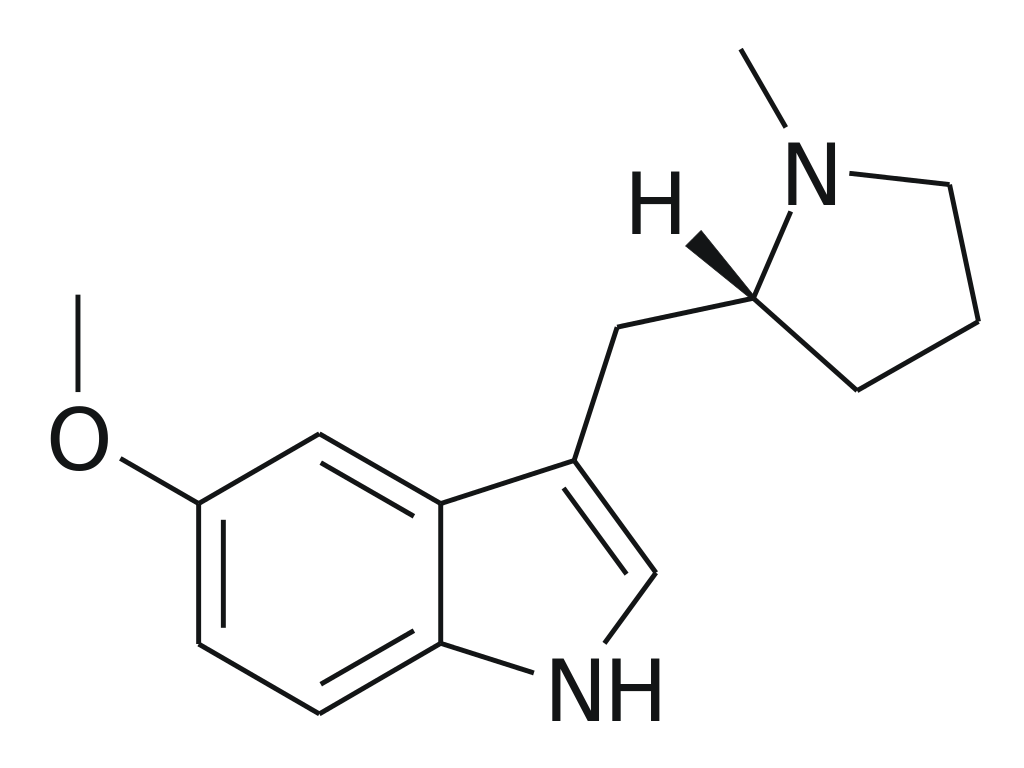
5-MeO-MPMI, scientifically referred to as 5-Methoxy-N-methyl-(α, N-trimethylene)tryptamine, is a derivative of tryptamine known for its psychedelic properties. The compound was initially synthesized in 1992 by a team led by JE Macor. Subsequently, in the late 1990s, it underwent investigations by a team led by David Nichols at Purdue University. In animal tests, this substance elicits responses consistent with psychedelics, demonstrating a potency similar to the amphetamine-derived psychedelic DOI. Notably, 5-MeO-MPMI has two enantiomers, of which only the (R)-enantiomer is active.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 143321-57-7 |
|---|---|
| PubChem CID | 9881324 |
| ChemSpider | 8057000 |
| UNII | N29TFW7VRV |
| ChEMBL | ChEMBL137485 |
| Chemical and physical data | |
| Formula | C15H20N2O |
| Molar mass | 244.338 g·mol−1 |
FAQ
1. What is 5-MeO-MPMI?
5-MeO-MPMI, also known as 5-Methoxy-N-methyl-(α, N-trimethylene)tryptamine, is a derivative of the tryptamine class of compounds. It is recognized for its psychedelic effects.
2. Who developed 5-MeO-MPMI, and when was it first synthesized?
5-MeO-MPMI was initially synthesized in 1992 by a research team led by JE Macor. It was later investigated by a team led by David Nichols at Purdue University in the late 1990s.
3. What are the psychedelic properties of 5-MeO-MPMI?
5-MeO-MPMI produces psychedelic effects, which can include alterations in perception, mood, and consciousness. These effects are similar to those induced by other psychedelic substances.
4. How does 5-MeO-MPMI compare to other psychedelics in terms of potency?
In animal tests, 5-MeO-MPMI demonstrates a potency level akin to the amphetamine-derived psychedelic DOI. This suggests that it can elicit potent psychedelic responses.
5. Are there different enantiomers of 5-MeO-MPMI, and does this impact its activity?
Yes, 5-MeO-MPMI has two enantiomers. However, only the (R)-enantiomer is active, meaning that its psychedelic effects are attributed to this specific form of the compound.
6. Is 5-MeO-MPMI legal and available for recreational use?
The legal status of 5-MeO-MPMI varies by country and region. It may be subject to specific drug regulations, and its availability for recreational use may be restricted. Always check your local laws and regulations.
7. Is 5-MeO-MPMI used for therapeutic purposes?
The use of 5-MeO-MPMI for therapeutic purposes has yet to be extensively studied. It is important to note that the therapeutic use of such substances should only occur under the guidance of qualified medical professionals and in a clinical setting.
8. Where can I find more information about 5-MeO-MPMI?
For more information on 5-MeO-MPMI, consider consulting scientific literature, academic research or speaking with healthcare professionals or experts in the field of psychoactive substances. Always prioritize safety and responsible use when considering the consumption of any such compounds.
References
- In 1992, Macor JE, Blake J, Fox CB, Johnson C, Koe BK, Lebel LA, Morrone JM, Ryan K, Schmidt AW, Schulz DW, and others conducted research, as documented in “Synthesis and serotonergic pharmacology of the enantiomers of 3-[(N-methylpyrrolidin-2-yl)methyl]-5-methoxy-1H-indole: discovery of stereogenic differentiation in the aminoethyl side chain of the neurotransmitter serotonin.” This study was published in the Journal of Medicinal Chemistry (Volume 35, Issue 23). The article’s DOI is doi:10.1021/jm00101a032, and it can be found under PMID 1447752.
- In 1999, Gerasimov M, Marona-Lewicka D, Kurrasch-Orbaugh DM, Qandil AM, and Nichols DE conducted further studies on oxygenated tryptamines with LSD-like activity. They incorporated a chiral pyrrolidine moiety into the side chain. This research was documented in “Further studies on oxygenated tryptamines with LSD-like activity incorporating a chiral pyrrolidine moiety into the side chain” and published in the Journal of Medicinal Chemistry (Volume 42, Issue 20). The document is accessible through CiteSeerX 10.1.1.690.4941, and the DOI is doi:10.1021/jm990325u. The study is referenced under PMID 10514296.