Summary
5-Methoxy-N,N-diisopropyltryptamine (often referred to as 5-MeO-DiPT, colloquially known as foxy methoxy or simply foxy) is a member of the psychedelic tryptamine class and represents the methoxy derivative of diisopropyltryptamine (DiPT).
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 4021-34-5 |
|---|---|
| PubChem CID | 151182 |
| DrugBank | DB01441 |
| ChemSpider | 133247 |
| UNII | 12D06G8W8E |
| ChEBI | CHEBI:48282 |
| CompTox Dashboard (EPA) | DTXSID00193209 |
| Chemical and physical data | |
| Formula | C17H26N2O |
| Molar mass | 274.408 g·mol−1 |
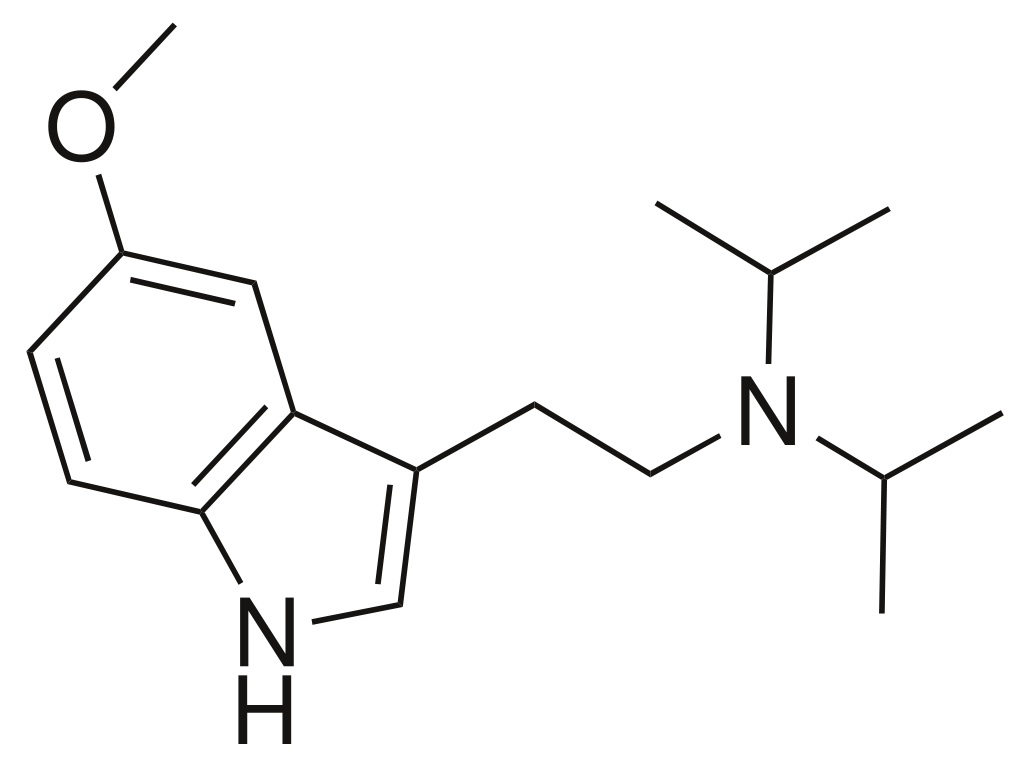
Pharmacology
The hallucinogenic and entheogenic effects of 5-MeO-DiPT are believed to be primarily generated through 5-HT2A receptor agonism. However, it’s worth noting that additional mechanisms of action, such as monoamine oxidase inhibition (MAOI), may also play a role. Notably, 5-MeO-DiPT exhibits the strongest receptor binding affinity for the 5-HT1A receptor.
Furthermore, it’s important to highlight that 5-MeO-DiPT has been found to be neurotoxic in rats.
Overdose
Excessive doses of 5-MeO-DiPT have led to clinical intoxication, manifesting as symptoms like nausea, vomiting, agitation, hypotension, mydriasis, tachycardia, and hallucinations, particularly in young adults. Several cases of overdose can be linked to the drug’s extended onset of action. First-time users, unacquainted with the substance, sometimes administered a second dose when they initially felt no effects. In one case, a young man experienced rhabdomyolysis and renal failure, while another individual tragically passed away 3 4 hours following an apparent rectal overdose. It’s noteworthy that at least one fatality has been associated with the consumption of 5-MeO-DiPT.
Drug prohibition laws
Here is the legal status of 5-MeO-DiPT in various countries:
- China: 5-MeO-DiPT became a controlled substance in China as of October 2015.
- Denmark: It has been illegal in Denmark since February 2004.
- Germany: The substance was deemed illegal in Germany since September 1999.
- Greece: 5-MeO-DiPT has been illegal in Greece since February 2003.
- Japan: It became illegal in Japan in April 2005.
- Singapore: 5-MeO-DiPT was declared illegal in Singapore in early 2006.
- Sweden: The health ministry of Sweden (Sveriges riksdags Statens folkhälsoinstitut) classified 5-MeO-DiPT as a “health hazard” under the Act on the Prohibition of Certain Goods Dangerous to Health (Lagen om förbud mot vissa hälsofarliga varor) as of October 1, 2004. It is listed as 5-metoxi-N,N-diisopropyltryptamin (5-MeO-DIPT), making it illegal to sell or possess.
- United States: In the United States, the DEA added both 5-MeO-DiPT and alpha-methyltryptamine (AMT) to Schedule I of the Controlled Substances Act on April 4, 2003, using “emergency scheduling” procedures. These substances were officially placed into Schedule I on September 29, 2004. Prior to its prohibition in the U.S., 5-MeO-DiPT was sold online, along with psychoactive analogues like DiPT and DPT, neither of which had been expressly outlawed at the time.
FAQ
- What is 5-MeO-DiPT?
- 5-Methoxy-N, N-diisopropyltryptamine (5-MeO-DiPT) is a psychedelic tryptamine compound. It is the methoxy derivative of diisopropyltryptamine (DiPT).
- How does 5-MeO-DiPT work?
- The primary mechanism behind the hallucinogenic and entheogenic effects of 5-MeO-DiPT is believed to be 5-HT2A receptor agonism. It may also interact with other receptors, such as 5-HT1A.
- Is 5-MeO-DiPT neurotoxic?
- Yes, 5-MeO-DiPT has been found to be neurotoxic in rat studies.
- What are the effects of 5-MeO-DiPT?
- 5-MeO-DiPT is known to produce psychedelic effects, which may include hallucinations, altered perception, and changes in mood. It can also lead to nausea, vomiting, agitation, hypotension, mydriasis (dilated pupils), tachycardia (rapid heart rate), and in some cases, hallucinations.
- What are the potential risks of 5-MeO-DiPT use?
- Excessive doses of 5-MeO-DiPT can lead to clinical intoxication with a range of adverse effects, including nausea, vomiting, and hallucinations. Overdoses, often due to the drug’s delayed onset of action, have been reported and can be life-threatening.
- Is 5-MeO-DiPT legal?
- The legal status of 5-MeO-DiPT varies by country. In some countries, it is a controlled substance, while in others, it may be classified as illegal or restricted. It is essential to be aware of the laws in your jurisdiction.
- Can 5-MeO-DiPT be used for therapeutic purposes?
- There is limited research on the therapeutic potential of 5-MeO-DiPT. Some anecdotal reports suggest its potential use in treating cluster headaches, but more research is needed to establish its efficacy and safety for therapeutic purposes.
- Where can I find 5-MeO-DiPT?
- The availability of 5-MeO-DiPT may vary by region and is subject to legal restrictions. It is important to be aware of the laws and regulations in your area before seeking or using this substance.
- Is 5-MeO-DiPT the same as other tryptamines?
- No, 5-MeO-DiPT is distinct from other tryptamines, and its effects and risks may differ from those of other substances in the same class.
- What precautions should I take when using 5-MeO-DiPT?
- If you choose to use 5-MeO-DiPT, it is crucial to be well informed about its effects, dosage, and potential risks. Always start with a low dose, and never use it alone. Ensure you are in a safe and controlled environment. Additionally, stay within the legal boundaries of your jurisdiction.
References
- Anvisa (2023-07-24). “RDC Nº 804 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial” [Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- Narimatsu S, Yonemoto R, Saito K, Takaya K, Kumamoto T, Ishikawa T, et al. (April 2006). “Oxidative metabolism of 5-methoxy-N,N-diisopropyltryptamine (Foxy) by human liver microsomes and recombinant cytochrome P450 enzymes”. Biochemical Pharmacology. 71 (9): 1377–1385. doi:10.1016/j.bcp.2006.01.015. PMID 16510126.
- Nagai F, Nonaka R, Satoh Hisashi Kamimura K (March 2007). “The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain”. European Journal of Pharmacology. 559 (2–3): 132–137. doi:10.1016/j.ejphar.2006.11.075. PMID 17223101.
- Ray TS (February 2010). “Psychedelics and the human receptorome”. PLOS ONE. 5 (2): e9019. Bibcode:2010PLoSO…5.9019R. doi:10.1371/journal.pone.0009019. PMC 2814854. PMID 20126400.
- Noworyta-Sokołowska K, Kamińska K, Kreiner G, Rogóż Z, Gołembiowska K (November 2016). “Neurotoxic Effects of 5-MeO-DIPT: A Psychoactive Tryptamine Derivative in Rats”. Neurotoxicity Research. 30 (4): 606–619. doi:10.1007/s12640-016-9654-0. PMC 5047954. PMID 27461536.
- Baselt R (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. pp. 975–976.
- Tanaka E, Kamata T, Katagi M, Tsuchihashi H, Honda K (November 2006). “A fatal poisoning with 5-methoxy-N,N-diisopropyltryptamine, Foxy”. Forensic Science International. 163 (1–2): 152–154. doi:10.1016/j.forsciint.2005.11.026. PMID 16406422.
- “关于印发《非药用类麻醉药品和精神药品列管办法》的通知” (in Chinese). China Food and Drug Administration. 27 September 2015. Archived from the original on 1 October 2015. Retrieved 1 October 2015.
- “notisum.se” (PDF). Archived (PDF) from the original on 2013-09-29. Retrieved 2013-09-06.