Beautiful Plants For Your Interior
Summary
Morphine belongs to the morphinan class of naturally occurring opioid substances. It is one of the alkaloids naturally found in opium, derived from the poppy plant Papaver somniferum, alongside codeine. Furthermore, morphine serves as the prototype opiate, setting the standard against which other opioids are measured, including codeine, diacetylmorphine (commonly known as heroin), and hydrocodone.
The isolation of morphine from opium is credited to Friedrich Sertürner, a German pharmacist’s assistant, who achieved this breakthrough in 1806.
Morphine’s subjective effects encompass sedation, pain relief, cough suppression, and euphoria. However, it carries potentially severe side effects, notably respiratory depression and low blood pressure, which can prove fatal when combined with other depressants. Additional side effects include drowsiness, vomiting, and constipation.
The primary source of morphine production involves isolating it from the poppy straw of the opium poppy plant.
Morphine can be administered through various routes, including oral, intramuscular, subcutaneous, intravenous, spinal cord space injection, or rectal administration. Intravenous administration produces its maximum effect in approximately 20 minutes, while oral ingestion takes around 60 minutes. The duration of its effects typically ranges from three to seven hours. Extended-release formulations, like Kadian, can maintain clinically significant effects for up to 24 hours.
It is widely acknowledged that morphine possesses a significant potential for abuse. Apart from the risk of fatal overdose from single-use, chronic morphine use leads to increased tolerance and physical and psychological dependence, posing substantial harm to the user. Therefore, it is strongly recommended to adhere to harm-reduction practices when using this substance.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 57-27-2 64-31-3 (neutral sulfate), 52-26-6 (hydrochloride) |
|---|---|
| PubChem CID | 5288826 |
| IUPHAR/BPS | 1627 |
| DrugBank | DB00295 |
| ChemSpider | 4450907 |
| UNII | 76I7G6D29C |
| KEGG | D08233 |
| ChEBI | CHEBI:17303 |
| ChEMBL | ChEMBL70 |
| PDB ligand | MOI (PDBe, RCSB PDB) |
| CompTox Dashboard (EPA) | DTXSID9023336 |
| ECHA InfoCard | 100.000.291 |
| Chemical and physical data | |
| Formula | C17H19NO3 |
| Molar mass | 285.343 g·mol−1 |
History and culture
In 1806, German pharmacist’s assistant Friedrich Sertürner achieved a groundbreaking milestone by isolating morphine for the first time.
Throughout more than 50 experiments, Sertürner believed he had successfully extracted the primary active component from opium, marking a pivotal moment as it is generally considered the earliest isolation of an active compound from a plant. This active substance, found to be ten times as potent as opium, was initially named “morphium” by Sertürner, drawing inspiration from Morpheus, the Greek god of dreams, due to its propensity to induce sleep. Merck subsequently commenced commercial marketing of morphine in 1827. While the broader medical community did not immediately recognize the full clinical significance of morphine, it was not until 1831 that Sertürner’s contribution was duly acknowledged, and he received the French equivalent of the Nobel Prize. As the opium plant underwent further analysis, other alkaloids were identified, with one eventually becoming known as codeine. The term “codeine” traces its origins to the ancient Greek word “κώδεια,” referring to a cup shaped like a poppyhead.
The widespread use of morphine was significantly influenced by Dr. Alexander Wood’s invention of the hypodermic syringe in 1853. However, it wasn’t until the American Civil War in 1861, followed by the Prussian-Austrian War in 1866 and the Franco-Prussian War of 1870, that morphine saw extensive application in the field of military medicine. It was during and after these military conflicts that morphine habituation acquired the ominous moniker of the “soldier’s disease” or the “army disease.” Recognizing the addictive and habit-forming nature of morphine, the medical community began searching for a less addictive alternative, which eventually led to the introduction of diacetylmorphine, later marketed as heroin. Ironically, it was later discovered that heroin was not less addictive than codeine but rather the opposite.
The first proposed chemical structure of morphine emerged in 1923, a hypothesis that was subsequently confirmed in 1927 by Robinson and colleagues. Finally, the complete chemical synthesis of morphine was achieved in 1952 by Gates and Tschudi.
Morphine’s primary source is derived from the poppy straw of the opium poppy plant. In 2013, an estimated 523,000 kilograms of morphine were produced, with approximately 45,000 kilograms being directly employed for pain management—an increase fourfold over the past two decades. The majority of this medical usage is concentrated in the developed world. Notably, roughly 70% of morphine serves as a precursor for the production of other opioids like hydromorphone, oxycodone, and heroin.
Chemistry
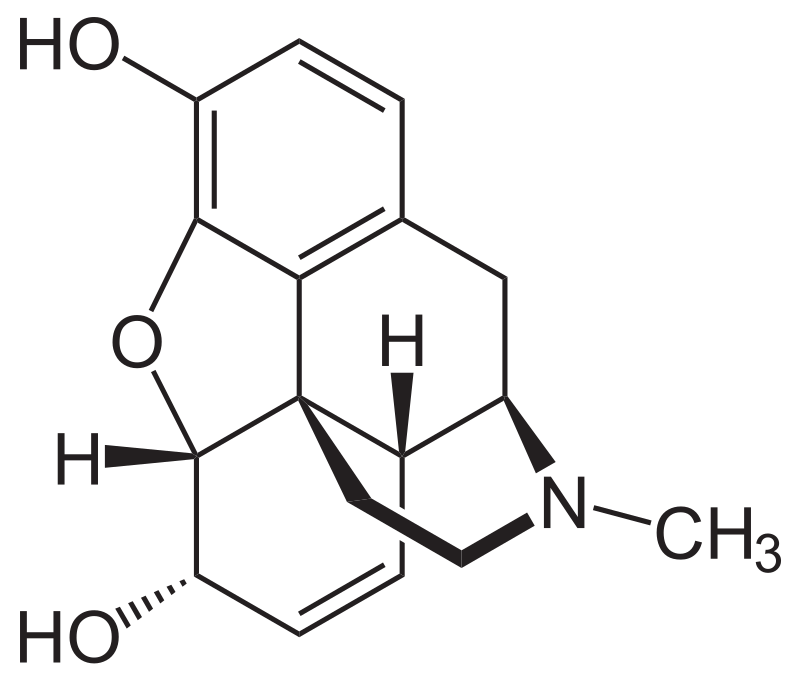
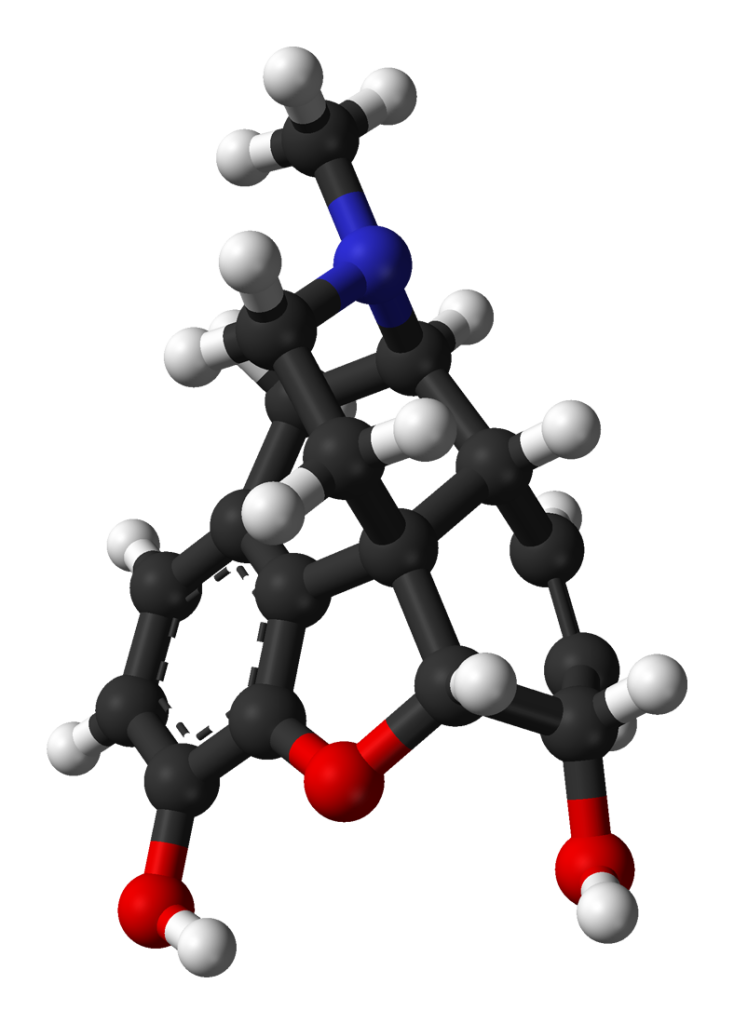
Morphine, classified as a benzylisoquinoline alkaloid, belongs to the morphinan class of opiates. In this chemical family, morphine and its counterparts share a distinctive structural core characterized by three interconnected benzene rings arranged in a zig-zag pattern known as phenanthrene. Additionally, a fourth ring containing nitrogen is fused to the phenanthrene structure at positions R9 and R13, with the nitrogen element situated at position R17 within the combined framework. This fused structure is referred to as a “morphinan.”
Morphine, along with other substances in the morphinan class, features an ether bridge linking two of its rings, specifically connecting positions R4 and R5 through an oxygen group. It possesses two hydroxy groups (OH-) attached at positions R6 and R3 and a methyl group located on the nitrogen atom at position R17.
Morphine serves as a precursor for numerous morphinan-based drugs, playing a pivotal role in the synthesis of codeine through methylation of its hydroxy group at position R3 and in the production of heroin through acetylation. Other closely related opioids, such as hydrocodone, oxycodone, and dihydrocodeine, share structural similarities with morphine. The fundamental chemical structure of morphine serves as the foundational framework for the development of hundreds of opioid derivatives, each with a diverse range of effects.
Pharmacology
Morphine exerts its effects by activating three well-known opioid receptors: the κ-opioid (KOP), μ-opioid (MOP), and δ-opioid (DOP) receptors, acting as an agonist. This mechanism of action is rooted in the functional mimicry of the body’s natural endorphins. Endorphins play a crucial role in pain reduction (analgesia), inducing sleepiness and generating sensations of pleasure and enjoyment. These endorphins are typically released in response to various stimuli, including pain, strenuous physical exercise, orgasm, or moments of excitement. By emulating the action of these natural endorphins, morphine delivers its characteristic euphoric, analgesic (pain relief), and anxiolytic (anti-anxiety) effects.
In small amounts, morphine is produced within the human body and serves as an immunomodulator. The endogenous morphine primarily binds to the μ3 opioid receptor.
Metabolism of morphine, hydromorphone, and oxymorphone involves minimal CYP450 enzymatic activity and is instead predominantly facilitated by the action of UDP-glucuronosyltransferases (UGTs), specifically the UG2B7 isozyme. Approximately 90% of morphine undergoes conversion into metabolites, primarily manifesting as glucuronide conjugates: morphine-3-glucuronide (M3G) (45-55%) and morphine-6-glucuronide (M6G) (10-15%). Among these metabolites, M3G exhibits low affinity for opioid receptors and lacks analgesic activity, while M6G binds to the same receptor sites as morphine but exhibits higher affinity and potency.
Subjective effects
Please note that the effects listed below are based on anecdotal user reports and the personal analyses of contributors to the Subjective Effect Index (SEI) on PsychonautWiki. They should be approached with a degree of skepticism, as individual experiences can vary widely, and higher doses are more likely to produce a broader range of effects. Additionally, it’s crucial to be aware that adverse effects become more likely with higher doses and may include addiction, severe injury, or even death ☠.
Physical:
- Sedation
- Intense physical euphoria – Morphine’s physical euphoria is notably intense, surpassing that of opioids like codeine or tramadol. It induces profound feelings of physical comfort, warmth, love, and bliss.
- Muscle relaxation
- Constipation
- Cough suppression
- Decreased libido
- Difficulty urinating
- Itchiness
- Nausea
- Pain relief – While users may still be aware of pain, it often becomes less bothersome.
- Pupil constriction
- Respiratory depression
- Skin flushing
- Appetite suppression
- Orgasm suppression
Cognitive:
- Cognitive euphoria – Morphine generates a cognitive euphoria that is significantly stronger than that induced by opioids like codeine or tramadol but milder than that of heroin, hydrocodone, or oxycodone. It results in powerful and all-encompassing feelings of emotional bliss, contentment, and happiness.
- Anxiety suppression
- Thought deceleration
- Compulsive redosing
- Dream potentiation
- Irritability – While opioids are known for their mood-enhancing properties, they can paradoxically increase sensitivity to irritants. This may lead to aloofness and sudden outbursts of anger and aggression, often referred to as “opiate rage.” This effect tends to occur more frequently during the comedown or with heavy use.
Visual:
- Suppressions
- Double vision – At high doses, the eyes may unfocus and refocus uncontrollably, resulting in a blurred and double vision that persists regardless of where one looks. This effect can become so intense that reading or driving becomes impossible.
- Hallucinatory states
- Internal hallucination – During heavy nodding from high doses, users may enter a state of semi-consciousness and hypnagogia, leading to dream-like states and up to level 3 imagery. Ill-defined geometric patterns often accompany this experience.
Toxicity
Similar to most opioids, pure morphine generally doesn’t lead to many long-term complications, except for the development of dependence and constipation. The potential dangers associated with morphine use primarily stem from improper administration, overdosing, or the use of impure products.
Using high doses of morphine can result in respiratory depression, which can progress to life-threatening oxygen deprivation (anoxia). This happens because morphine’s interaction with µ-opioid receptors suppresses the natural breathing reflex, with the degree of suppression increasing with higher doses.
Morphine can also cause nausea and vomiting, and some opioid overdose-related deaths occur due to unconscious individuals aspirating on their vomit. This happens when someone unconscious or semi-conscious and lying on their back vomits into their mouth, unknowingly causing suffocation. This risk can be mitigated by ensuring the person lies on their side with their head tilted downwards, a position known as the recovery position.
It is strongly advised to practice harm reduction when using morphine.
Dependence and Abuse Potential:
Like other opiate-based painkillers, chronic morphine use is highly addictive and can lead to both physical and psychological dependence. When physical dependence develops, abrupt discontinuation can lead to withdrawal symptoms.
Tolerance to many of morphine’s effects, including its therapeutic effects, typically develops with prolonged use. This results in users needing progressively larger doses to achieve the same effects. The development of tolerance to the constipation-inducing effects is particularly slow. Importantly, morphine use can lead to cross-tolerance with other opioids, meaning that after using morphine, other opioids become less effective.
The risk of fatal opioid overdoses significantly increases after a period of abstinence and relapse due to reduced tolerance. To address this, it is safer to take only a fraction of one’s usual dosage if relapsing. Additionally, the environment in which the drug is taken can influence opioid tolerance; studies have shown that familiarity with the environment can impact tolerance levels.
Dangerous Interactions:
Morphine should not be combined with other depressants, as many reported overdose cases involve interactions with substances such as alcohol or benzodiazepines. These interactions can lead to dangerously high levels of respiratory depression.
Warning: Combining psychoactive substances, even those considered safe on their own, can become dangerous and life-threatening when mixed with certain other substances. Conduct independent research to ensure the safety of combining two or more substances. Some known dangerous interactions include:
- Alcohol: Both alcohol and morphine potentiate each other’s ataxia and sedation, potentially causing loss of consciousness, vomiting, and memory blackouts.
- Amphetamines: Stimulants can increase respiration rate, allowing for higher opioid doses. If the stimulant wears off first, the opioid may lead to respiratory arrest.
- Benzodiazepines: These central nervous system depressants can potentiate morphine’s effects, rapidly causing unconsciousness. Unconscious users are at risk of vomit aspiration, with blackouts and memory loss likely.
- Cocaine: Stimulants like cocaine increase respiration rate, potentially allowing for higher opioid doses. However, if the stimulant wears off first, the opioid can lead to respiratory arrest.
- DXM: This combination is generally considered toxic, with risks including CNS depression, difficulty breathing, heart issues, and liver toxicity. DXM may also reduce opioid tolerance.
- GHB/GBL: These substances potentiate each other unpredictably, leading to rapid unconsciousness. Unconscious individuals may be at risk of vomit aspiration.
- Ketamine: Both substances carry risks of vomiting and unconsciousness, especially if the user becomes unconscious under their influence.
- MAOIs: Combining MAOIs with opioids can lead to severe adverse reactions, including agitation, seizures, and even death in some cases.
- MXE: This substance can potentiate opioid effects but also increases the risk of respiratory depression and organ toxicity.
- Nitrous: Combining nitrous oxide and morphine can potentiate ataxia and sedation, potentially leading to unconsciousness and vomit aspiration.
- PCP: PCP may reduce opioid tolerance, increasing the risk of overdose.
- Tramadol: There’s an increased risk of seizures when combining tramadol with other opioids. Central nervous system and respiratory-depressant effects may also be additive or synergistic.
- Grapefruit: While not psychoactive itself, grapefruit can affect the metabolism of certain opioids, potentially increasing their toxicity and duration of action. This effect varies depending on the specific opioid and its metabolic pathway.
Legal status
Internationally, morphine is categorized as a Schedule I substance in accordance with the Single Convention on Narcotic Drugs. Furthermore, it holds a place on the WHO Model List of Essential Medicines, which comprises crucial medications essential for a basic healthcare system.
Here is the regulatory status of morphine in several countries:
- Australia: Morphine is designated as a Schedule 8 drug, subject to the laws of different Australian states and territories, as outlined in their respective Poisons Acts.
- Austria: Morphine is legal for medical purposes under the AMG (Arzneimittelgesetz Österreich), but it is illegal to sell or possess it without a prescription, in compliance with the SMG (Suchtmittelgesetz Österreich).
- Canada: Morphine is classified as a Schedule I substance under the Controlled Drugs and Substances Act.
- France: Morphine falls under the strictest category of controlled substances, in accordance with the French controlled substances law enacted in December 1970.
- Germany: Morphine is a controlled substance listed under Anlage III of the BtMG. It can only be prescribed on a narcotic prescription form.
- Japan: Morphine is categorized as a narcotic under the Narcotic and Psychotropic Drugs Control Act (麻薬及び向精神薬取締法).
- Netherlands: Morphine is classified as a List 1 drug, regulated by the Opium Law.
- Russia: Morphine is a Schedule II controlled substance.
- Sweden: Morphine is legally available for medical use but is classified as a controlled substance.
- Switzerland: Morphine is a controlled substance explicitly listed under Verzeichnis A. Medicinal use is permitted.
- Turkey: Morphine is categorized as a ‘red prescription’ only substance, and it is illegal to sell or possess it without a prescription.
- United Kingdom: Morphine is categorized as a Class A drug under the Misuse of Drugs Act 1971 and is considered a Schedule 2 Controlled Drug according to the Misuse of Drugs Regulations 2001.
- United States: Morphine is classified as a Schedule II controlled substance under the Controlled Substances Act, with the main Administrative Controlled Substances Code Number (ACSCN) of ACSCN 9300. In the U.S., morphine pharmaceuticals are subject to annual manufacturing quotas. However, morphine production for use in highly diluted formulations and as a chemical precursor for other drug synthesis is excluded from these manufacturing quotas.
FAQ
1. What is morphine?
- Morphine is a powerful opioid medication commonly used to relieve severe pain. It is derived from the opium poppy plant and is known for its potent analgesic (pain-relieving) properties.
2. How does morphine work?
- Morphine works by binding to specific receptors in the brain and spinal cord called opioid receptors. This action blocks pain signals and produces feelings of relaxation and euphoria.
3. What is morphine used for?
- Morphine is primarily used to manage severe pain, such as that experienced after surgery, injury, or in cancer patients. It is also used in palliative care to alleviate pain in patients with terminal illnesses.
4. Is morphine addictive?
- Yes, morphine has a high potential for addiction and dependence, both physical and psychological. It should only be used under the close supervision of a healthcare provider and as prescribed.
5. What are the common side effects of morphine?
- Common side effects may include drowsiness, constipation, nausea, vomiting, and itching. Some people may also experience dizziness or difficulty concentrating.
6. Can I drink alcohol while taking morphine?
- It is not recommended to consume alcohol while taking morphine. Both substances can depress the central nervous system and may lead to dangerous side effects, including respiratory depression.
7. How should I take morphine?
- Morphine is usually taken orally in the form of tablets or liquid. Follow your healthcare provider’s instructions carefully, and do not crush or chew extended-release tablets.
8. Can I drive or operate heavy machinery while on morphine?
- It is generally not safe to drive or operate heavy machinery while taking morphine, as it can impair your judgment and coordination. Always follow your healthcare provider’s advice regarding activities while on this medication.
9. Is morphine safe during pregnancy and breastfeeding?
- Morphine should only be used during pregnancy or breastfeeding under the guidance of a healthcare professional. It can potentially pass into breast milk and harm the baby.
10. What should I do if I miss a dose of morphine? – If you miss a dose, take it as soon as you remember unless it’s close to the time for your next scheduled dose. In that case, skip the missed dose and continue with your regular dosing schedule. Do not double up on doses.
11. Can morphine be used to treat addiction to other opioids? – Yes, in certain cases, healthcare providers may use morphine or other opioid agonists to help manage opioid addiction and withdrawal symptoms as part of a medically supervised treatment program.
12. Is morphine available over-the-counter (OTC)? – No, morphine is not available over-the-counter. It is a prescription medication and should only be used under the guidance of a healthcare provider.
13. What should I do if I suspect an overdose or have severe side effects? – If you suspect an overdose or experience severe side effects such as difficulty breathing, extreme drowsiness, or loss of consciousness, seek immediate medical attention. In case of overdose, naloxone can be administered to reverse the effects of morphine.
14. How long does the pain relief from morphine last? – The duration of pain relief from morphine varies depending on the formulation and dosage. Immediate-release morphine provides relief for about 4 to 6 hours, while extended-release forms may provide pain relief for up to 12 or 24 hours.
15. Can I stop taking morphine abruptly? – It is important not to stop taking morphine suddenly, especially if you have been using it for an extended period, as this can lead to withdrawal symptoms. Consult your healthcare provider for guidance on tapering off the medication safely.
References
- Introduction: This resource explores the risks associated with combining depressant drugs, highlighting the importance of understanding potential interactions and their effects on the central nervous system.
- Source References: This information draws from various scientific and medical sources, including pharmacological reviews, research articles, and government publications.
- Mechanism of Action: Depressant drugs, including morphine, act on the central nervous system by slowing down its activity. This can lead to relaxation, pain relief, and sedation.
- Metabolism and Effects: Morphine undergoes metabolism in the body, leading to the formation of various metabolites. These metabolites can have different effects on opioid receptors and contribute to the overall pharmacological profile of morphine.
- Risk of Addiction: Morphine has a high potential for both physical and psychological dependence, making it crucial to use it only as prescribed by a healthcare professional.
- Common Side Effects: When taking morphine, individuals may experience side effects such as drowsiness, constipation, nausea, vomiting, and itching.
- Alcohol Interaction: Combining alcohol with morphine is not recommended, as both substances can depress the central nervous system, potentially leading to dangerous side effects.
- Driving and Machinery Operation: It is generally unsafe to operate vehicles or heavy machinery while taking morphine, as it can impair judgment and coordination.
- Pregnancy and Breastfeeding: Morphine use during pregnancy and breastfeeding should be closely monitored by healthcare professionals due to potential risks to the baby.
- Missed Doses: If a dose of morphine is missed, it should be taken as soon as possible unless it’s close to the next scheduled dose. Doubling up on doses is not recommended.
- Tapering Off: To discontinue morphine safely, individuals should consult with a healthcare provider to develop a tapering plan to prevent withdrawal symptoms.
- Overdose and Severe Side Effects: Suspected overdose or severe side effects, such as difficulty breathing or loss of consciousness, require immediate medical attention. Naloxone may be administered to reverse morphine’s effects in overdose cases.
- Global Regulatory Status: The legal status of morphine varies by country, with some nations classifying it as a controlled substance and others permitting its medical use under specific regulations.
- International Control: Morphine is subject to international control under agreements like the Single Convention on Narcotic Drugs.
- Essential Medicine: Morphine is included in the WHO Model List of Essential Medicines, emphasizing its importance in healthcare systems.
- Conclusion: Understanding the risks and potential interactions of morphine is crucial for safe and responsible use. Always consult with a healthcare provider for personalized guidance on morphine use.