Contents
Summary
O-Desmethyltramadol, also referred to as O-DSMT or desmetramadol, belongs to the phenylpropylamine class of opioid substances. This compound serves as an active metabolite originating from tramadol.
Before emerging for sale in the research chemical market during the 2010s, O-DSMT had not been documented for human consumption.
Subjectively, individuals who use O-DSMT report experiencing effects such as sedation, pain relief, anxiety reduction, muscle relaxation, and a sense of euphoria. In contrast to tramadol, O-DSMT is often described as less stimulating and closer in sensation to traditional opiates. Due to its role as the primary contributor to tramadol’s pain-relieving properties, O-DSMT is notably more potent by weight than its parent compound.
Despite its analgesic potential, O-DSMT has a relatively brief history of human use, and there is limited knowledge about its toxicity and the risks of abuse. It is strongly recommended that individuals exercise harm reduction strategies if considering the use of this substance.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 80456-81-1 |
|---|---|
| PubChem CID | 130829 |
| ChemSpider | 115703 |
| UNII | 2WA8F50C3F |
| ChEMBL | ChEMBL1400 |
| CompTox Dashboard (EPA) | DTXSID40894102 |
| Chemical and physical data | |
| Formula | C15H23NO2 |
| Molar mass | 249.354 g·mol−1 |
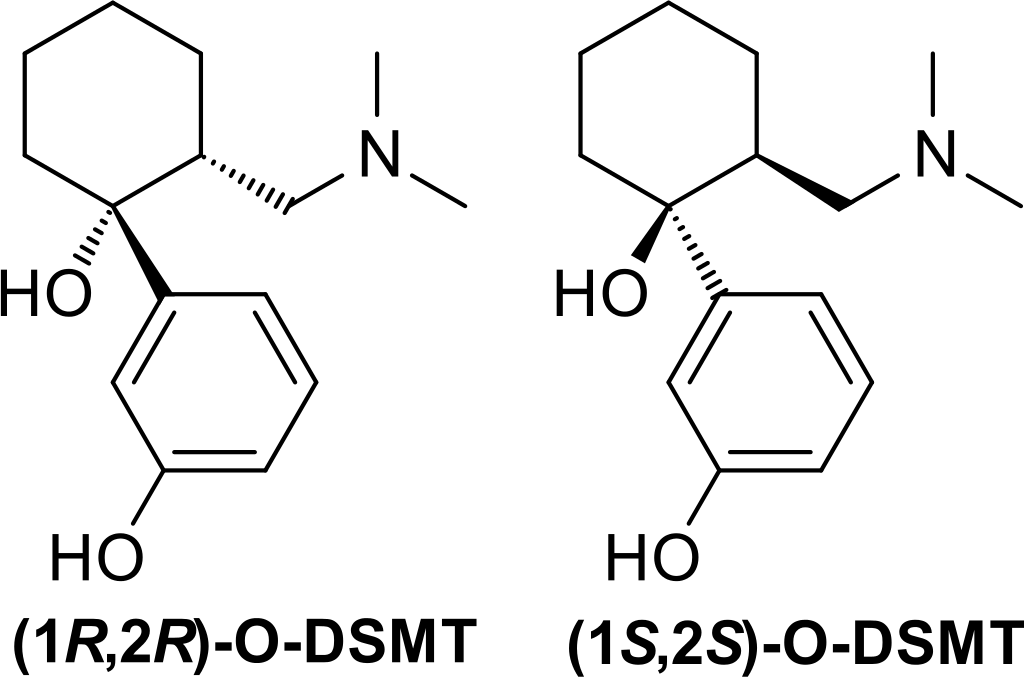
Chemistry
O-Desmethyltramadol, also known as O-DMST, is an unusual synthetic opioid compound. It differs from traditional morphinan opiates and bears a loose resemblance to codeine. Instead of belonging to the morphinan class, it possesses a unique chemical structure characterized by two interconnected rings: a cyclohexane ring fused to a phenyl ring at R1. This phenyl ring is further substituted with a hydroxy group (OH-) at R3. Another hydroxy group is located at the same position where the cyclohexane ring connects to the phenyl ring, R1. Additionally, O-DMST carries a third substitution on its cyclohexane ring at R2, with the ring being linked to a dimethylamine group through a methylene bridge.
What makes O-Desmethyltramadol atypical is that it occurs as a racemate, comprising a combination of its stereoisomers. Stereoisomers are two molecules sharing the same chemical formula but having distinct three-dimensional structures as mirror images of each other. Tramadol is synthesized as a racemate of its two isomers because this combination is known to be more effective. Flipping the orientation of the R2 and R1 bonds results in the formation of the R- and S-enantiomers of O-Desmethyltramadol. O-DMST closely resembles tramadol, and its name reflects the absence of the methyl group found in tramadol’s R3 methoxy substitution.
Pharmacology
O-DSMT exhibits significantly greater potency as a μ-opioid agonist when compared to tramadol. Furthermore, in contrast to tramadol, O-DSMT serves as a high-affinity ligand for both δ- and κ-opioid receptors.
The two enantiomers of O-DSMT demonstrate distinct pharmacological characteristics, with both the (+) and (−)-O-DSMT forms lacking serotonin reuptake inhibitory activity. However, (−)-O-DSMT retains its capacity as a norepinephrine reuptake inhibitor, contributing to the intricate pharmacological profile of tramadol when combined with its parent compound and metabolites. While the diverse receptor interactions can be advantageous in pain management, particularly for complex conditions like neuropathic pain, they heighten the potential for drug interactions compared to other opioids and may also lead to side effects.
Opioids exert their effects by binding to and activating μ-opioid receptors. This mechanism is possible because opioids structurally resemble naturally occurring endorphins found in the body, which also act on μ-opioid receptors. The structural resemblance to these endorphins accounts for the euphoria, pain relief, and anxiety reduction induced by opioids. Endorphins play a role in pain reduction, drowsiness, and the elicitation of pleasurable sensations. They can be released in response to various stimuli such as pain, strenuous physical activity, orgasm, or general excitement.
Subjective effects
Disclaimer: The effects detailed below are based on the Subjective Effect Index (SEI), which relies on open research literature, anecdotal user accounts, and personal assessments by contributors to PsychonautWiki. As a result, it is advisable to approach these effects with a measure of skepticism.
Furthermore, it should be noted that these effects may not manifest consistently or predictably, with higher doses being more likely to encompass the full spectrum of effects. Conversely, increased doses also heighten the risk of adverse outcomes, which may include addiction, severe harm, or even death ☠.
Physical:
- O-DSMT can induce a general sensation characterized by intense euphoria, profound relaxation, anxiety alleviation, and pain relief.
- Pain relief
- Physical euphoria: The physical euphoria elicited by this substance is considered less intense when compared to that of morphine or diacetylmorphine (heroin). It manifests as an extreme sense of physical comfort, warmth, affection, and bliss.
- Itchiness
- Respiratory depression: At lower to moderate doses, this effect entails a mild to moderate slowing down of breathing without causing significant impairment. However, high doses and overdoses may lead to opioid-induced respiratory depression, resulting in shortness of breath, abnormal breathing patterns, semi-consciousness, or unconsciousness. Severe overdoses can lead to coma or fatality without immediate medical attention.
- Constipation
- Cough suppression
- Difficulty urinating
- Nausea
- Sedation
- Pupil constriction
- Decreased libido
- Appetite suppression
- Orgasm suppression
Cognitive:
- Cognitive euphoria: The cognitive euphoria induced by this substance is considered less intense when compared to that of morphine or diacetylmorphine (heroin). It entails a powerful and overwhelming sense of emotional bliss, contentment, and happiness.
- Anxiety suppression
- Compulsive redosing
- Dream potentiation
Toxicity
- Alcohol: Combining O-DSMT with alcohol can potentiate the sedative and ataxic effects of both substances, potentially leading to sudden loss of consciousness at high doses. Patients affected by this combination should be placed in the recovery position to prevent aspiration from excessive vomiting. Memory blackouts are likely.
- Amphetamines: Stimulants like amphetamines can increase the rate of respiration, allowing for higher opioid doses. However, if the stimulant’s effects wear off before the opioid, respiratory arrest may occur.
- Benzodiazepines: Combining benzodiazepines with O-DSMT can lead to additive central nervous system and respiratory depressant effects. These substances can potentiate each other rapidly and unpredictably, possibly resulting in unconsciousness. While unconscious, there is a risk of vomit aspiration, and blackouts or memory loss are common.
- Cocaine: Stimulants like cocaine can increase respiration rate, permitting higher opioid doses. However, if the stimulant wears off before the opioid, respiratory arrest may occur.
- DXM: DXM is generally considered toxic when combined with opioids. This combination can lead to central nervous system depression, breathing difficulties, heart problems, and liver toxicity. Additionally, DXM can slightly reduce opioid tolerance, leading to additional synergistic effects.
- GHB/GBL: These substances can strongly and unpredictably potentiate each other, rapidly inducing unconsciousness. While unconscious, there is a risk of vomit aspiration if the individual is not placed in the recovery position.
- Ketamine: Both substances pose a risk of vomiting and unconsciousness. Suppose a user falls unconscious while under the influence of this combination; there is a severe risk of vomit aspiration if they are not placed in the recovery position.
- MAOIs: Combining monoamine oxidase inhibitors (MAOIs) with certain opioids has been associated with severe adverse reactions. These interactions can be excitatory or depressive, leading to symptoms such as agitation, headache, sweating, fever, flushing, shivering, seizures, and even coma. Death has occurred in some cases.
- MXE: MXE can potentiate the effects of opioids while increasing the risk of respiratory depression and organ toxicity.
- Nitrous: Both substances can potentiate each other’s ataxic and sedative effects, potentially leading to sudden loss of consciousness at high doses. While unconscious, there is a risk of vomit aspiration, and memory blackouts are common.
- PCP: PCP may reduce opioid tolerance, increasing the risk of overdose.
- Tramadol: Combining O-DSMT with tramadol may increase the risk of seizures. Tramadol itself is known to induce seizures, and this combination may have additive effects on seizure threshold. Central nervous system and respiratory depressant effects can also be additive or synergistic.
- Grapefruit: Although not psychoactive, grapefruit can affect the metabolism of certain opioids, including tramadol, oxycodone, and fentanyl. Grapefruit juice potently inhibits the enzyme CYP3A4, which is responsible for metabolizing these opioids, leading to prolonged drug clearance. This can increase the risk of toxicity with repeated doses. Methadone may also be affected. Codeine and hydrocodone, metabolized by CYP2D6, may not work in individuals who lack the enzyme due to a genetic mutation or drug interaction.
Serotonin Syndrome Risk: Combining O-DSMT with the following substances can lead to dangerously high serotonin levels, potentially causing serotonin syndrome, which requires immediate medical attention and can be fatal if untreated:
- MAOIs: Such as banisteriopsis caapi, Syrian rue, phenelzine, selegiline, and moclobemide.
- Serotonin releasers: Such as MDMA, 4-FA, methamphetamine, methylone, and αMT.
- SSRIs: Such as citalopram and sertraline.
- SNRIs: Such as tramadol and venlafaxine.
- 5-HTP
Legal status
Information regarding the international legal status of O-Desmethyltramadol possession is limited, but it has been confirmed as a controlled substance in the United Kingdom.
- Germany: O-DSMT is not classified as a controlled substance under the BtMG. It is considered legal as long as it is not marketed for human consumption, in accordance with §2 AMG.
- Sweden: O-DSMT is classified as a controlled substance and falls under narcotic regulations.
- Switzerland: O-DSMT is not listed under Buchstabe A, B, C, or D in controlled substance regulations, suggesting it may be considered legal.
- United Kingdom: The production, supply, or importation of this drug is illegal under the Psychoactive Substance Act, which became effective on May 26, 2016.
FAQ
1. What is Desmetramadol (O-DSMT)? Desmetramadol, often referred to as O-DSMT, is a synthetic opioid substance. It is an active metabolite of tramadol, a commonly prescribed pain medication.
2. How does O-DSMT differ from tramadol? O-DSMT is a metabolite of tramadol, and it is considered more potent by weight when it comes to its analgesic (pain-relieving) effects. It also has a different pharmacological profile and is often compared to traditional opiates. Unlike tramadol, O-DSMT is not a controlled substance in all countries, which can lead to differences in its legal status.
3. What are the effects of O-DSMT? O-DSMT can produce various effects, including pain relief, euphoria, sedation, anxiety suppression, and muscle relaxation. These effects are common with opioid substances. However, it is important to note that the effects can vary from person to person.
4. Is O-DSMT safe to use? The safety of O-DSMT use is a subject of concern. Due to its limited history of human use, not much is known about its long-term effects, toxicity, and potential for abuse. Additionally, O-DSMT can be dangerous when combined with other depressant substances like alcohol or benzodiazepines.
5. Is O-DSMT legal? The legal status of O-DSMT varies from country to country. In some nations, it is considered a controlled substance and is illegal to possess or distribute. In others, it may not be explicitly regulated, but it’s essential to research and understand the specific laws in your jurisdiction.
6. What precautions should I take if I choose to use O-DSMT? If you decide to use O-DSMT, it’s crucial to practice harm reduction. Start with a low dose, avoid mixing it with other substances, and be aware of potential side effects. Never use it in a way that wasn’t intended or prescribed by a medical professional.
7. Can O-DSMT lead to addiction? Like other opioids, O-DSMT has the potential for abuse and addiction, especially with chronic use. Users may develop tolerance, leading to the need for higher doses to achieve the same effects. Quitting O-DSMT after developing an addiction can lead to withdrawal symptoms.
8. What are some dangerous interactions with O-DSMT? Combining O-DSMT with certain substances, such as alcohol, benzodiazepines, or other opioids, can lead to life-threatening interactions. Always research potential interactions and exercise caution when using O-DSMT with other substances.
9. Where can I find more information about O-DSMT? While information about O-DSMT may be limited, you can consult reputable sources, harm reduction organizations, and medical professionals for guidance. Remember to verify the legal status of O-DSMT in your region before considering its use.
10. Is O-DSMT available for medical use? O-DSMT is not typically prescribed for medical purposes. Its status as a controlled substance or legality may restrict its use in medical settings. Medical decisions regarding pain management should be made by healthcare professionals based on individual patient needs and legal regulations.
References
- Sevcik, J., Nieber, K., Driessen, B., Illes, P. (September 1993). “Effects of the central analgesic tramadol and its main metabolite, O-desmethyltramadol, on rat locus coeruleus neurones”. British Journal of Pharmacology. 110 (1): 169–176. doi:10.1111/j.1476-5381.1993.tb13788.x. ISSN 0007-1188.
- Dayer, P., Desmeules, J., Collart, L. (1997). “[Pharmacology of tramadol]”. Drugs. 53 Suppl 2: 18–24. doi:10.2165/00003495-199700532-00006. ISSN 0012-6667.
- Potschka, H., Friderichs, E., Löscher, W. (September 2000). “Anticonvulsant and proconvulsant effects of tramadol, its enantiomers and its M1 metabolite in the rat kindling model of epilepsy”. British Journal of Pharmacology. 131 (2): 203–212. doi:10.1038/sj.bjp.0703562. ISSN 0007-1188.
- Bamigbade, T. A., Davidson, C., Langford, R. M., Stamford, J. A. (September 1997). “Actions of tramadol, its enantiomers and principal metabolite, O-desmethyltramadol, on serotonin (5-HT) efflux and uptake in the rat dorsal raphe nucleus”. British Journal of Anaesthesia. 79 (3): 352–356. doi:10.1093/bja/79.3.352. ISSN 0007-0912.
- Driessen, B., Reimann, W., Giertz, H. (March 1993). “Effects of the central analgesic tramadol on the uptake and release of noradrenaline and dopamine in vitro”. British Journal of Pharmacology. 108 (3): 806–811. doi:10.1111/j.1476-5381.1993.tb12882.x. ISSN 0007-1188.
- Arndt, T., Claussen, U., Güssregen, B., Schröfel, S., Stürzer, B., Werle, A., Wolf, G. (20 May 2011). “Kratom alkaloids and O-desmethyltramadol in urine of a “Krypton” herbal mixture consumer”. Forensic Science International. 208 (1–3): 47–52. doi:10.1016/j.forsciint.2010.10.025. ISSN 1872-6283.
- Bäckstrom, B. G., Classon, G., Löwenhielm, P., Thelander, G. (15 December 2010). “[Krypton–new, deadly Internet drug. Since October 2009 have nine young persons died in Sweden]”. Lakartidningen. 107 (50): 3196–3197. ISSN 0023-7205.
- Kronstrand, R., Roman, M., Thelander, G., Eriksson, A. (1 May 2011). “Unintentional Fatal Intoxications with Mitragynine and O-Desmethyltramadol from the Herbal Blend Krypton”. Journal of Analytical Toxicology. 35 (4): 242–247. doi:10.1093/anatox/35.4.242. ISSN 0146-4760.
- Ershad, M., Cruz, M. D., Mostafa, A., Mckeever, R., Vearrier, D., Greenberg, M. I. (March 2020). “Opioid Toxidrome Following Grapefruit Juice Consumption in the Setting of Methadone Maintenance”. Journal of Addiction Medicine. 14 (2): 172–174. doi:10.1097/ADM.0000000000000535. ISSN 1932-0620.
- Gillman, P. K. (2005). “Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity”. British Journal of Anaesthesia. 95 (4): 434–441. doi:10.1093/bja/aei210 Freely accessible. eISSN 1471-6771. ISSN 0007-0912. OCLC 01537271. PMID 16051647.
- UK Legislation
- Anlage I BtMG – Einzelnorm
- § 2 AMG – Einzelnorm
- Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien
- Psychoactive Substances Act 2016