Summary
AB-PINACA, originally detected as an ingredient in synthetic cannabis products in Japan back in 2012, has a notable history. Initially, it was formulated by Pfizer in 2009 with the intent of serving as an analgesic medication.
This compound exhibits a strong affinity for the CB1 receptor (Ki = 2.87 nM, EC50 = 1.2 nM) and CB2 receptor (Ki = 0.88 nM, EC50 = 2.5 nM). In rat discrimination studies, AB-PINACA effectively mimics the effects of Δ9-THC, albeit with 1.5 times greater potency.
However, it’s crucial to acknowledge that the use of AB-PINACA has been linked to various cases of fatalities and hospitalizations, underscoring the potential risks associated with this synthetic cannabinoid.
| Identifiers | |
|---|---|
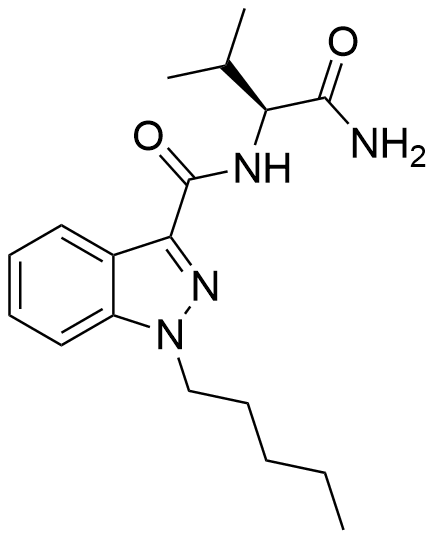
| IUPAC name | |
| CAS Number | 1445752-09-9 |
|---|---|
| PubChem CID | 71301472 |
| ChemSpider | 28537615 |
| UNII | 6J3KC3S2PA |
| CompTox Dashboard (EPA) | DTXSID00904034 |
| Chemical and physical data | |
| Formula | C18H26N4O2 |
| Molar mass | 330.432 g·mol−1 |
Legal status
Germany:
AB-PINACA has been categorized as an Anlage II controlled substance in Germany since November 2014.
Singapore:
AB-PINACA is classified in the Fifth Schedule of the Misuse of Drugs Act, rendering it illegal in Singapore, effective May 2015.
United States:
AB-PINACA is designated as a Schedule I controlled substance in the United States, subject to stringent regulations.
China:
As of October 2015, AB-PINACA is acknowledged as a controlled substance in China, with possession and use prohibited.
France:
In March 2017, AB-PINACA was classified as a controlled substance in France, subject to legal restrictions and enforcement.
FAQ
1. What is AB-PINACA?
- AB-PINACA is a synthetic compound that was initially developed as a potential analgesic medication but has been associated with synthetic cannabis products.
2. How was AB-PINACA discovered?
- AB-PINACA was first identified as a component of synthetic cannabis products in Japan in 2012.
3. What are the pharmacological properties of AB-PINACA?
- AB-PINACA is a potent agonist for the CB1 and CB2 receptors, with an affinity for these receptors and potency comparable to Δ9-THC. It is approximately 1.5 times more potent than Δ9-THC in certain studies.
4. Are there any health risks associated with AB-PINACA use?
- Yes, the use of AB-PINACA has been linked to various reported cases of deaths and hospitalizations, indicating potential health risks.
5. What is the legal status of AB-PINACA in different countries?
- AB-PINACA is classified as an Anlage II controlled substance in Germany. It is illegal in Singapore, the United States (as a Schedule I controlled substance), China, and France, each with its legal restrictions and penalties.
6. Is AB-PINACA still used for medical purposes?
- No, AB-PINACA was initially developed by Pfizer as a potential analgesic medication but is not approved for medical use.
7. Where can I find more information about the risks and regulations related to AB-PINACA?
- To obtain further information about the risks and legal status of AB-PINACA, it is advisable to consult local health authorities, substance abuse professionals, or relevant drug education resources. Staying informed is essential for making responsible choices.
References
- Anvisa, the Brazilian Health Regulatory Agency, issued Collegiate Board Resolution No. 804 on July 24, 2023, addressing the control of substances with narcotic, psychotropic, precursor, and other special characteristics in Brazil. It provides crucial information about such substances under special control. [Reference: Anvisa (2023-07-24). “RDC Nº 804 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial” [Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.]
- In 2012, Uchiyama and colleagues discovered AB-PINACA as a new cannabimimetic indazole derivative, marking its identification as a designer drug in illegal products. [Reference: Uchiyama N, Matsuda S, Wakana D, Kikura-Hanajiri R, Goda Y (2012). “New cannabimimetic indazole derivatives, N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide (AB-PINACA) and N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide (AB-FUBINACA) identified as designer drugs in illegal products”. Forensic Toxicology. 31: 93–100. doi:10.1007/s11419-012-0171-4. S2CID 25242453.]
- Cayman Chemical is a reputable source of information about AB-PINACA, offering valuable insights into this synthetic compound. [Reference: “AB-PINACA”. Cayman Chemical. Retrieved 25 June 2015.]
- AB-PINACA’s origins can be traced back to a patent issued to Pfizer Inc. in 2009. This patent describes indazole derivatives, providing valuable historical context. [Reference: WO 2009106980A, Buchler IP, Hayes MJ, Hegde SG, Hockerman SL, Jones DE, Kortum SW, Rico JG, Tenbrink RE, Wu KK, “Indazole derivatives”, published 3 September 2009, assigned to Pfizer Inc.]
- Banister and colleagues conducted research in September 2015, examining the pharmacology of various synthetic cannabinoid designer drugs, including AB-PINACA. Their study sheds light on the pharmacological properties of these substances. [Reference: Banister SD, Moir M, Stuart J, Kevin RC, Wood KE, Longworth M, et al. (September 2015). “Pharmacology of Indole and Indazole Synthetic Cannabinoid Designer Drugs AB-FUBINACA, ADB-FUBINACA, AB-PINACA, ADB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, ADBICA, and 5F-ADBICA”. ACS Chemical Neuroscience. 6 (9): 1546–1559. doi:10.1021/acschemneuro.5b00112. PMID 26134475.]
- Wiley and colleagues investigated the affinity and potency of synthetic cannabinoids, including AB-PINACA, in September 2015, elucidating their effects in mice and their comparability to Δ9-tetrahydrocannabinol (THC). [Reference: Wiley JL, Marusich JA, Lefever TW, Antonazzo KR, Wallgren MT, Cortes RA, et al. (September 2015). “AB-CHMINACA, AB-PINACA, and FUBIMINA: Affinity and Potency of Novel Synthetic Cannabinoids in Producing Δ9-Tetrahydrocannabinol-Like Effects in Mice”. The Journal of Pharmacology and Experimental Therapeutics. 354 (3): 328–339. doi:10.1124/jpet.115.225326. PMC 4538877. PMID 26105953.]
- The New England Journal of Medicine published a study in July 2015 that explores synthetic cannabinoid-related illnesses and deaths, shedding light on potential health risks associated with these substances. [Reference: Trecki J, Gerona RR, Schwartz MD (July 2015). “Synthetic Cannabinoid-Related Illnesses and Deaths”. The New England Journal of Medicine. 373 (2): 103–107. doi:10.1056/NEJMp1505328. PMID 26154784.]
- In September 2015, Thornton and colleagues reported a case of unintentional pediatric exposure to AB-PINACA, resulting in a coma and intubation, highlighting the dangers associated with this synthetic cannabinoid. [Reference: Thornton SL, Akpunonu P, Glauner K, Hoehn KS, Gerona R (September 2015). “Unintentional Pediatric Exposure to a Synthetic Cannabinoid (AB-PINACA) Resulting in Coma and Intubation”. Annals of Emergency Medicine. 66 (3): 343–344. doi:10.1016/j.annemergmed.2015.05.021. PMID 26304261.]
- The legal status of AB-PINACA in Germany is provided by the Gesetz über den Verkehr mit Betäubungsmitteln (Betäubungsmittelgesetz – BtMG), specifically categorized under Anlage II. This highlights its controlled status within the country. [Reference: “Gesetz über den Verkehr mit Betäubungsmitteln (Betäubungsmittelgesetz – BtMG) Anlage II (zu § 1 Abs. 1) (verkehrsfähige, aber nicht verschreibungsfähige Betäubungsmittel)” [Law on the Traffic in Narcotic Substances (Narcotics Act – BtMG)