Summary
ADB-CHMINACA, also recognized as MAB-CHMINACA, belongs to the class of indazole-based synthetic cannabinoids. Initially designed by Pfizer in 2009 for its potential as an analgesic medication, it stands out as a robust agonist of the CB1 receptor, boasting an impressive binding affinity with a Ki value as low as 0.289 nM. This compound gained notoriety when it was detected in cannabinoid mixtures in Japan in early 2015.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1185887-13-1 |
|---|---|
| PubChem CID | 68894304 |
| ChemSpider | 48059556 |
| UNII | SL4C60689M |
| Chemical and physical data | |
| Formula | C21H30N4O2 |
| Molar mass | 370.497 g·mol−1 |
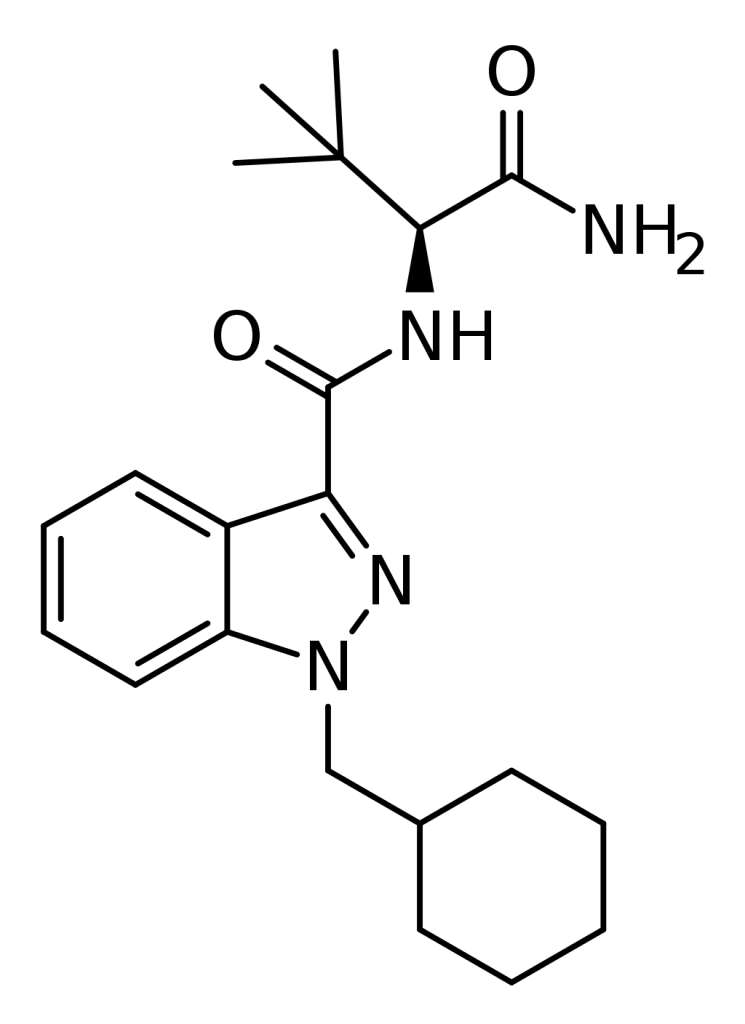
Side effects
Numerous reported cases link this synthetic cannabinoid to fatalities and hospital admissions, emphasizing its potential dangers.
Legal Status
In the United States, ADB-CHMINACA has been classified as a Schedule I controlled substance. Notably, before its federal scheduling in 2018, Louisiana had placed ADB-CHMINACA on its Schedule I list through emergency scheduling in 2014.
On November 10, 2014, Sweden’s public health agency proposed categorizing ADB-CHMINACA as a hazardous substance.
As of May 2015, ADB-CHMINACA is categorized as illegal in Singapore due to its inclusion in the Fifth Schedule of the Misuse of Drugs Act (MDA).
In Switzerland, the substance was declared illegal in December 2015.
Metabolism
Extensive research has identified ten major metabolites of ADB-CHMINACA in various incubations involving cryopreserved human hepatocytes. Most of these metabolic transformations primarily occur in the cyclohexylmethyl tail of the compound.
FAQ
1. What is ADB-CHMINACA?
- ADB-CHMINACA is a synthetic cannabinoid, also known as a designer drug. It’s chemically designed to mimic the effects of natural cannabinoids found in cannabis.
2. What are the effects of ADB-CHMINACA?
- ADB-CHMINACA can induce various effects, including psychoactive and mood-altering experiences. However, it’s essential to note that these effects can be unpredictable and potentially harmful.
3. Is ADB-CHMINACA legal?
- The legality of ADB-CHMINACA varies by country and jurisdiction. In many places, it is classified as a controlled substance due to its potential for misuse and associated risks.
4. What are the health risks associated with ADB-CHMINACA use?
- ADB-CHMINACA use is linked to several health risks, including addiction potential, adverse psychological effects, and potential harm to physical health. Long-term consequences can be severe.
5. Can ADB-CHMINACA be detected in drug tests?
- Yes, ADB-CHMINACA can be detected in drug tests designed to identify synthetic cannabinoids. These tests are becoming more sophisticated in detecting such substances.
6. Why have there been reported cases of deaths and hospitalizations related to ADB-CHMINACA?
- ADB-CHMINACA is associated with these cases due to its potent and sometimes unpredictable effects, as well as the varying purity and dosage of the substance found in illicit products.
7. What is the legal status of ADB-CHMINACA in my country?
- The legal status of ADB-CHMINACA can differ widely from one country to another. It is crucial to check with local authorities or regulatory agencies for the most current information on its legal status.
8. How can I seek help if I or someone I know is using ADB-CHMINACA?
- Suppose you or someone you know is using ADB-CHMINACA and needs assistance. In that case, it’s essential to seek help from a medical professional, addiction counselor, or a local substance abuse support group.
9. Can ADB-CHMINACA be used for medicinal purposes?
- No, ADB-CHMINACA is not approved for medical use. It is considered a designer drug and is not used for any therapeutic purposes.
10. What is the metabolism of ADB-CHMINACA?
- The metabolism of ADB-CHMINACA has been extensively studied, and several major metabolites have been identified in human hepatocytes. Most of the metabolic transformations occur in the cyclohexylmethyl tail of the compound.
11. Is ADB-CHMINACA related to natural cannabis?
- ADB-CHMINACA is a synthetic cannabinoid designed to mimic the effects of natural cannabinoids. Still, it is a distinct chemical compound created for recreational use rather than a naturally occurring substance.
References
- Anvisa, on July 24, 2023, published “RDC Nº 804,” known as “Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control” in Brazilian Portuguese. This publication was archived from the original on August 27, 2023, and retrieved on the same date.
- For valuable insights into MAB-CHMINACA, refer to information provided by Cayman Chemical, retrievable from their records dated June 16, 2015.
- WO 2009/106980 holds information on “Indazole Derivatives” and was published on September 3, 2009. This patent was assigned to Pfizer Inc. and includes contributions from Buchler IP, Hayes MJ, Hegde SG, Hockerman SL, Jones DE, Kortum SW, Rico JG, Tenbrink RE, and Wu KK.
- In July 2015, research by Wurita A, Hasegawa K, Minakata K, Gonmori K, Nozawa H, Yamagishi I, Watanabe K, and Suzuki O unveiled the identification and quantitation of 5-fluoro-ADB-PINACA and MAB-CHMINACA in dubious herbal products. This study is accessible in “Forensic Toxicology” (Volume 33, Issue 2) through DOI: 10.1007/s11419-015-0264-y and S2CID 207289143.
- A study conducted by Hasegawa K, Wurita A, Minakata K, Gonmori K, Nozawa H, Yamagishi I, and others in July 2015 delved into the postmortem distribution of MAB-CHMINACA in body fluids and solid tissues of a human cadaver. The findings can be accessed in “Forensic Toxicology” (Volume 33, Issue 2) through DOI: 10.1007/s11419-015-0272-y, PMC 4525191, and PMID 26257834.
- The New York Times reported on April 24, 2015, about the potent ‘Spice’ drug and its contribution to an increase in emergency room visits.
- The American Association of Poison Control Centers, on April 23, 2015, issued a warning regarding the reemergence of synthetic drugs.
- In a PDF report dated May 11, 2015, the Alabama Department of Public Health highlighted the bizarre and violent behaviors exhibited by synthetic cannabinoid users, along with a surge in hospitalizations after use.
- Drug Scouts reported on May 7, 2015, cases of deaths associated with ADB-CHMINACA.
- Trecki J, Gerona RR, and Schwartz MD, in July 2015, discussed “Synthetic Cannabinoid-Related Illnesses and Deaths” in “The New England Journal of Medicine” (Volume 373, Issue 2) through DOI: 10.1056/NEJMp1505328 and PMID 26154784.
- Huffington Post reported on May 8, 2015, a sudden and alarming spike in hospitalizations caused by synthetic marijuana.
- Adamowicz P and Gieroń J, in September 2016, discussed “Acute intoxication of four individuals following use of the synthetic cannabinoid MAB-CHMINACA” in “Clinical Toxicology” (Volume 54, Issue 8) through DOI: 10.1080/15563650.2016.1190016 and PMID 27227269, along with S2CID 23133855.
- In January 2018, the Drug Enforcement Administration extended the temporary placement of MAB–CHMINACA in Schedule I of the Controlled Substances Act, as documented in the “Federal Register” (Volume 83, Issue 20) on pages 4411–4412, with PMID 29461023.
- Learn about the ban on the new synthetic marijuana compound by Gov. Jindal and State Officials, as retrieved on June 16, 2015.
- On June 29, 2015, Folkhälsomyndigheten discussed the proposal to classify cannabinoids as hazardous substances.
- On July 24, 2015, the Central Narcotics Bureau (CNB) issued a news release regarding synthetic cannabinoids.
- Der Bundesrat issued the “Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien” related to controlled substances.
- The metabolism of ADB-CHMINACA was investigated by Carlier J, Diao X, Sempio C, and Huestis MA in March 2017. This research identified new metabolites in human hepatocytes and is documented in “The AAPS Journal” (Volume 19, Issue 2) through DOI: 10.1208/s12248-016-0037-5 and PMID 28070717.