Summary
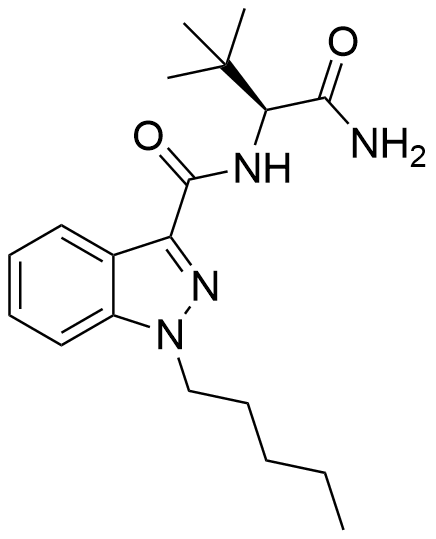
ADB-PINACA, a cannabinoid designer drug, finds its place as a constituent in certain synthetic cannabis products. It stands out as a robust agonist of both the CB1 and CB2 receptors, boasting impressive EC50 values of 0.52 nM and 0.88 nM, respectively. Notably, akin to MDMB-FUBINACA, this compound incorporates a tert-leucine amino acid residue
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1633766-73-0 |
|---|---|
| PubChem CID | 86280478 |
| ChemSpider | 29342129 |
| UNII | U1Q4F41C9U |
| CompTox Dashboard (EPA) | DTXSID301010007 |
| Chemical and physical data | |
| Formula | C19H28N4O2 |
| Molar mass | 344.459 g·mol−1 |
Side effects
ADB-PINACA usage has been associated with multiple cases of hospitalizations and fatalities.
Metabolism
Research has identified nineteen major metabolites of ADB-PINACA through various incubations with cryopreserved human hepatocytes. Key metabolic processes involve pentyl hydroxylation, hydroxylation with subsequent oxidation leading to ketone formation, and glucuronidation.
Legality
- ADB-PINACA is categorized as a controlled substance in Singapore, as it has been listed in the Fifth Schedule of the Misuse of Drugs Act (MDA) since May 2015.
- In the United States, ADB-PINACA is classified as a Schedule I controlled substance.[8] However, as of October 20th, 2023, its 5′-bromo analog, ADB-5’Br-PINACA, is not.
- Since October 2015, ADB-PINACA is considered a controlled substance in China.
FAQ
1. What is ADB-PINACA?
- ADB-PINACA is a synthetic cannabinoid commonly used in some synthetic cannabis products. It mimics the effects of natural cannabinoids found in the cannabis plant.
2. What are the health risks associated with ADB-PINACA use?
- ADB-PINACA has been linked to various health risks, including cases of hospitalizations and fatalities. These risks range from cardiovascular issues to neurological effects.
3. How does ADB-PINACA metabolize in the human body?
- Studies have identified nineteen major metabolites of ADB-PINACA, primarily occurring in human hepatocytes. The metabolic processes include pentyl hydroxylation, hydroxylation with oxidation (ketone formation), and glucuronidation.
4. Is ADB-PINACA legal?
- The legality of ADB-PINACA varies by country. In Singapore, it has been illegal since May 2015, as it is listed in the Fifth Schedule of the Misuse of Drugs Act (MDA). In the United States, it is classified as a Schedule I controlled substance. However, its 5′-bromo analog, ADB-5’Br-PINACA, is not considered a controlled substance as of October 20, 2023.
5. Are there analogs or related compounds to ADB-PINACA?
- Yes, there are analogs and related compounds. The synthetic cannabinoid family is known for its numerous variations and analogs that share similar properties.
6. What can individuals do if they or someone they know is using ADB-PINACA and needs help?
- Suppose you or someone you know is using ADB-PINACA and requires assistance. In that case, it’s crucial to seek help from a healthcare professional, addiction counselor, or a local substance abuse support group.
7. Why is ADB-PINACA considered a controlled substance in some countries?
- ADB-PINACA is categorized as a controlled substance due to its potential for abuse and associated health risks. It is regulated to protect public safety and health.
8. What is the difference between ADB-PINACA and its 5′-bromo analog, ADB-5’Br-PINACA, regarding legality?
- While ADB-PINACA is a controlled substance in the United States, its 5′-bromo analog, ADB-5’Br-PINACA, is not considered a controlled substance as of October 20, 2023. Legal distinctions can change over time.
9. Is ADB-PINACA related to natural cannabis?
- ADB-PINACA is a synthetic compound designed to replicate the effects of natural cannabinoids found in cannabis, but it is not derived from the cannabis plant. It is a product of synthetic drug design.
10. Are there ongoing studies or regulations related to ADB-PINACA?
- Regulatory and research efforts continue to monitor and address ADB-PINACA and other synthetic cannabinoids to understand their effects and potential risks better. It is essential to stay updated on any regulation changes or research findings.
References
- Banister SD, Moir M, Stuart J, Kevin RC, Wood KE, Longworth M, et al. uncovered the pharmacology of indole and indazole synthetic cannabinoid designer drugs, including AB-FUBINACA, ADB-FUBINACA, AB-PINACA, ADB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, ADBICA, and 5F-ADBICA in their study published in ACS Chemical Neuroscience.
- Learn more about ADB-PINACA from Forendex, an informative source for synthetic compounds, by accessing their archives.
- USA Today reported on a concerning outbreak of synthetic pot use in Colorado that led to 221 individuals falling ill, underlining the risks associated with synthetic cannabinoids like ADB-PINACA.
- A research study published in The Journal of Emergency Medicine by Schwartz MD and colleagues highlighted a common source outbreak of severe delirium linked to exposure to the novel synthetic cannabinoid ADB-PINACA, emphasizing the need for awareness and caution.
- In a publication in The New England Journal of Medicine, Trecki J, Gerona RR, and Schwartz MD discussed synthetic cannabinoid-related illnesses and deaths, shedding light on the potential dangers associated with these substances, including ADB-PINACA.[5]
- Carlier J, Diao X, Scheidweiler KB, and Huestis MA delved into the distinguishing features of new synthetic cannabinoids ADB-PINACA and 5F-ADB-PINACA using human hepatocyte metabolites and high-resolution mass spectrometry in their research, which was published in Clinical Chemistry.
- The Central Narcotics Bureau (CNB) issued a news release on April 30, 2015, concerning synthetic cannabinoids, providing valuable insights into the regulatory efforts related to these substances.
- The Drug Enforcement Administration (DEA) of the Department of Justice published a final order in the Federal Register in February 2014 regarding the temporary placement of four synthetic cannabinoids into Schedule I, highlighting their commitment to regulating these compounds.
- China Food and Drug Administration released a notice in Chinese on September 27, 2015, regarding the management of non-medical narcotic drugs and psychotropic drugs, emphasizing the significance of regulating these substances.