Beautiful Plants For Your Interior
Summary
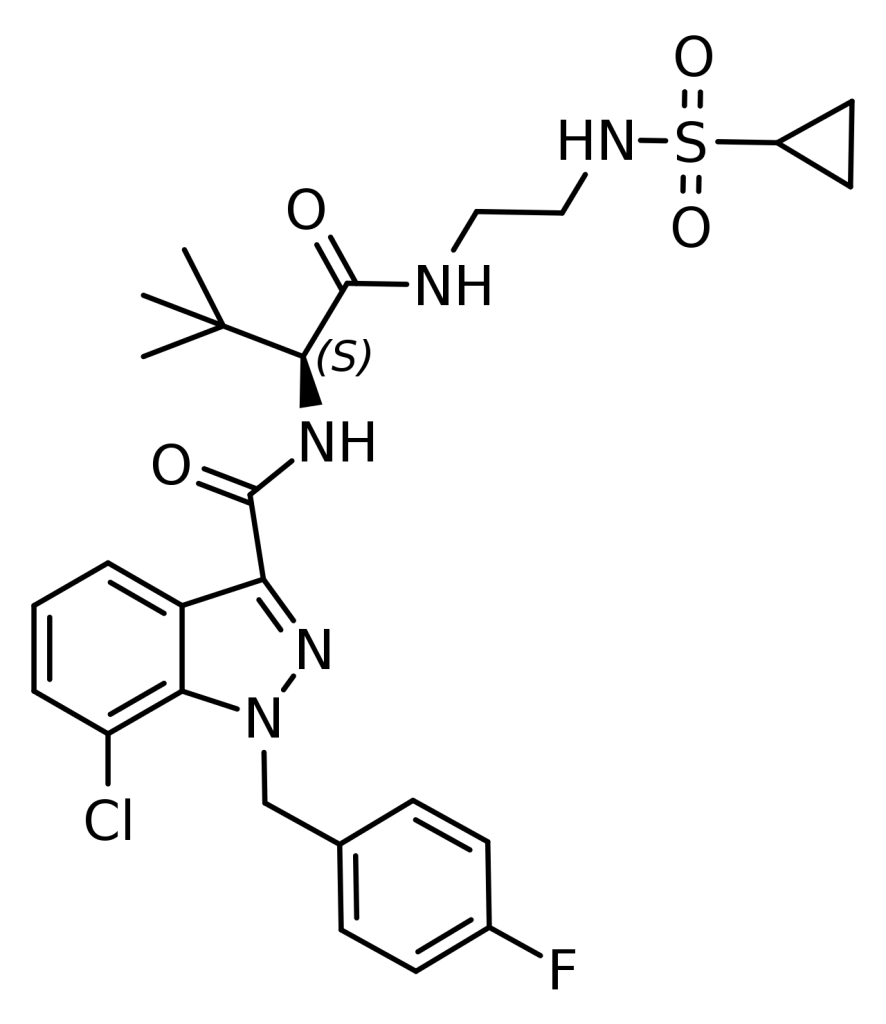
ADSB-FUB-187, an indazole-based synthetic cannabinoid, exhibits remarkable potency as a CB1 receptor agonist, boasting an impressively low binding affinity with a Ki value of 0.09 nM and an EC50 of 1.09 nM. Pfizer’s 2009 patent, WO 2009/106982, introduced this compound as example 187. While it stands out as the most tightly binding substance within this patent in terms of Ki, it’s essential to note that it doesn’t rank as the most potent compound in terms of producing a CB1-mediated pharmacological effect. At least 17 other compounds from the same patent exhibit lower EC50 values in this regard.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1185283-97-9 |
|---|---|
| PubChem CID | 44186812 |
| ChemSpider | 65322246 |
| UNII | L6K9PBL5EO |
| Chemical and physical data | |
| Formula | C26H31ClFN5O4S |
| Molar mass | 564.07 g·mol−1 |
Legality
On November 10, 2014, Sweden’s public health agency recommended the classification of ADSB-FUB-187 as a hazardous substance, prompted by its inclusion in unofficial synthetic cannabis products within the grey market.
FAQ
1. What is ADSB-FUB-187?
- ADSB-FUB-187 is a synthetic cannabinoid known for its potent agonistic activity at the CB1 receptor, a part of the endocannabinoid system in the human body.
2. How was ADSB-FUB-187 developed?
- ADSB-FUB-187 was originally developed by Pfizer in 2009 and is referred to as Example 187 in patent WO 2009/106982.
3. What is the binding affinity of ADSB-FUB-187 to the CB1 receptor?
- ADSB-FUB-187 exhibits a remarkable binding affinity with a Ki value of 0.09 nM, indicating its strong interaction with the CB1 receptor.
4. What is the pharmacological potency of ADSB-FUB-187?
- While highly binding, ADSB-FUB-187 is not the most potent compound for producing CB1-mediated pharmacological effects. At least 17 other compounds from the same patent have lower EC50 values in this regard.
5. Why was ADSB-FUB-187 considered for classification as a hazardous substance?
- Sweden’s public health agency suggested classifying ADSB-FUB-187 as a hazardous substance due to its presence as an ingredient in grey-market synthetic cannabis products, which raised concerns about its safety and potential health risks.
6. Is ADSB-FUB-187 legal?
- The legal status of ADSB-FUB-187 can vary by country, and it may be subject to regulations or restrictions. It’s essential to check your local laws and regulations regarding this substance.
7. What are the potential health risks associated with ADSB-FUB-187 use?
- The health risks associated with ADSB-FUB-187 use may include adverse effects on the endocannabinoid system, as well as risks related to synthetic cannabinoid consumption. These risks can range from psychological effects to physical health concerns.
8. Is ADSB-FUB-187 still in use today?
- The availability and use of synthetic cannabinoids like ADSB-FUB-187 may vary over time and by location. It’s crucial to stay informed about the current status and legal regulations in your area.
9. Are there ongoing studies or regulations related to ADSB-FUB-187?
- Research and regulatory efforts continue to monitor synthetic cannabinoids like ADSB-FUB-187 to understand their properties and potential risks better. It’s important to stay updated on any regulation changes or research findings.
References
- In a comprehensive study by Banister and Connor in 2018, the chemistry and pharmacology of synthetic cannabinoid receptor agonists, including new psychoactive substances, were explored. This research delves into the evolving landscape of these compounds, shedding light on their properties and effects.
- Patent WO 2009106982, authored by Buchler, Hayes, Hedge, Landis, Hockerman, Jones, Kortum, Rico, Tenbrink, and Wu, introduced the concept of indazole derivatives in 2009. The patent, assigned to Pfizer Inc., marks a pivotal moment in the development of synthetic cannabinoids.
- The Swedish Public Health Agency proposed the classification of cannabinoids as hazardous to health in 2015. This initiative reflects growing concerns about the potential risks associated with these substances, prompting regulatory considerations to safeguard public well-being.