Contents
Summary
AH-7921, an opioid analgesic drug, exhibits selectivity for the μ-opioid receptor, boasting approximately 90% of the potency of morphine when administered orally. This compound was initially uncovered during the 1970s by a research team at Allen and Hanburys, situated in the United Kingdom.
Distinguished as a new psychoactive substance (NPS), AH-7921 is meticulously crafted in laboratories to mimic the properties of controlled substances. Since 2012, this synthetic substance has been available for purchase on the internet, often masquerading under the alternative name “doxylamine” (not to be confused with doxylamine).
It is noteworthy that AH-7921 has never advanced to clinical trials, and the Drug Enforcement Administration (DEA) in the United States has yet to identify any medical applications for this substance. Consequently, the DEA has yet to prompt the Health and Human Services Department (HHS) to research the potential uses of AH-7921.
| Identifiers | |
|---|---|
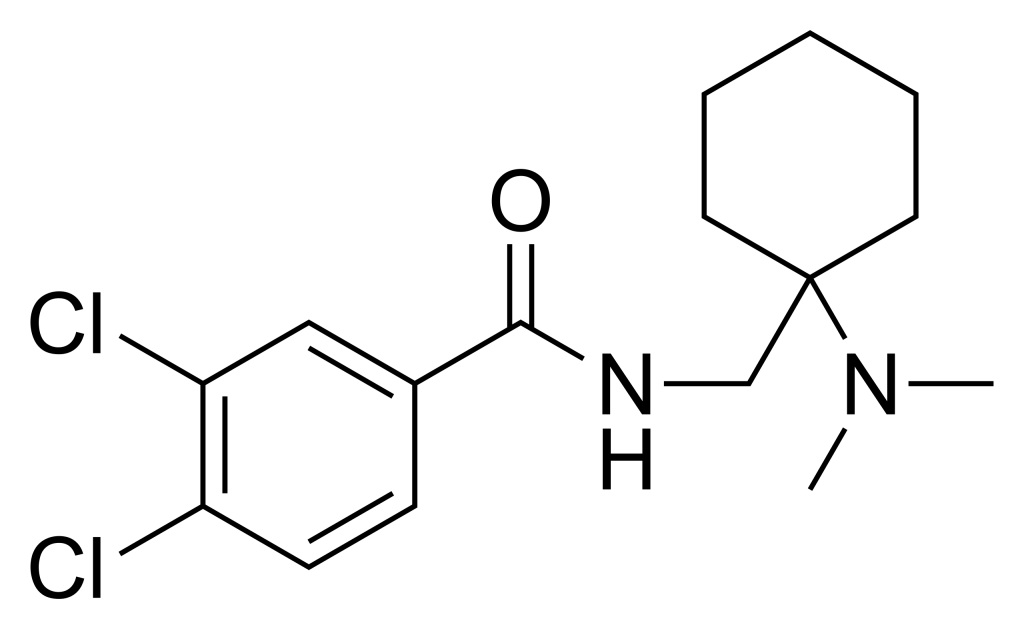
| IUPAC name | |
| CAS Number | 55154-30-8 |
|---|---|
| PubChem CID | 187760 |
| ChemSpider | 163208 |
| UNII | 10BR7A0SO0 |
| CompTox Dashboard (EPA) | DTXSID70203662 |
| Chemical and physical data | |
| Formula | C16H22Cl2N2O |
| Molar mass | 329.27 g·mol−1 |
Types of administration
- Intravenous Injection
- Nasal Insufflation
- Oral or Rectal Intake (when in the form of a powder, tablet, or capsule)[6]
- Sublingual Application
Side effects and withdrawal
- User Experience: Typically, AH-7921 doses range from 10 to 150 mg, leading users to encounter effects reminiscent of substances like heroin, morphine, and fentanyl. This often includes feelings of euphoria and respiratory depression.
- Overdose: In cases of overdose, users often exhibit symptoms such as tachycardia, hypertension, and seizures. The substance’s potency, shown in tests involving mice, dogs, and monkeys, nearly matches that of morphine, with a steep dose-response curve.
- Withdrawal: Rats administered 20 mg doses of AH-7921 three times a day for five days displayed withdrawal symptoms typical of opioids. Users have reported experiencing depression and insomnia when withdrawing from this drug.
Chemical Composition
- Physical Properties: AH-7921 is typically found as an off-white solid with a melting point in the range of 215–216°C. It consists of a single covalently bonded unit with four rotatable bonds. Additionally, it presents two hydrogen bond acceptors and one hydrogen bond donor.
USE
- Medical Use: Although extensively studied in vitro and animals by its developing company, AH-7921 was never commercially sold for medical purposes. It was discovered as an active ingredient in “synthetic cannabis” products in Japan in 2013.
- Racetrack Incidents: In October 2015, two horses (Bossmon and Literata) tested positive for AH-7921 at Belmont Park racetrack. These incidents raised concerns about its use in the racing industry.
- International Use: Norway and Iceland use AH-7921 as an analytical reference standard, but labels are mandated to state that it is not for human consumption to comply with the law.
- Fatalities: Multiple fatalities have been associated with AH-7921, with the substance linked to deaths in several countries. Postmortem examinations often revealed pulmonary edema as a common finding, with deaths occurring at both low and high concentrations of AH-7921 in femoral blood.
Legal Status
- Australia: AH-7921 was designated as a Prohibited Substance in Australia in May 2014 but later repealed, although it may still be a banned import.
- Israel: Distribution of AH-7921 has been illegal in Israel since December 2013.
- United Kingdom: In January 2015, AH-7921 was classified as a Class A drug in the UK under The Misuse of Drugs Act 1971 (Amendment) (No. 2) Order 2014.
- Brazil: AH-7921 has been illegal in Brazil since May 2015.
- China: As of October 2015, AH-7921 is considered a controlled substance in China.
- Czech Republic: AH-7921 is banned in the Czech Republic.
- United States: AH-7921 was placed in Schedule I of the Controlled Substances Act on May 16, 2016, due to its lack of medical use. Anyone seeking to conduct activities involving AH-7921 must be registered with the Drug Enforcement Administration.
- Canada: In Canada, the Controlled Drugs and Substances Act was amended in 2016 to include AH-7921 as a Schedule I substance. Possession without legal authority can lead to a maximum 7-year imprisonment.
Additionally, Health Canada amended the Food and Drug Regulations in May 2016 to classify AH-7921 as a restricted drug. Only those with specific authorization may possess the drug in Canada.
FAQ
1. What is AH-7921?
AH-7921 is an opioid analgesic drug that primarily targets the μ-opioid receptor. It is chemically designed to have a potency similar to morphine when taken orally.
2. How was AH-7921 discovered?
AH-7921 was first discovered in the 1970s by a research team at Allen and Hanburys, a company based in the United Kingdom.
3. Is AH-7921 a new psychoactive substance (NPS)?
Yes, AH-7921 is classified as a new psychoactive substance. It is synthetically created in laboratories to mimic the effects of controlled substances.
4. How is AH-7921 typically administered?
AH-7921 can be administered in various ways, including intravenous injection, nasal insufflation, oral or rectal ingestion (in powder, tablet, or capsule form), and sublingual application.
5. What are the effects of AH-7921 use?
Users commonly experience effects akin to those of heroin, morphine, and fentanyl, such as euphoria and respiratory depression. However, overdoses may lead to symptoms like tachycardia, hypertension, and seizures.
6. Are there withdrawal symptoms associated with AH-7921?
Yes, users who have taken AH-7921 and then discontinued it may experience withdrawal symptoms similar to other opioids. These symptoms can include depression and insomnia.
7. Is AH-7921 used for medical purposes?
AH-7921 was extensively studied in vitro and animals but was never commercially sold for medical use. The Drug Enforcement Administration (DEA) is not aware of any medical applications in the United States.
8. What is the legal status of AH-7921 in different countries?
The legal status of AH-7921 varies by country. In the United States, it is classified as a Schedule I controlled substance. Other countries, such as Australia, Israel, the United Kingdom, Brazil, China, and the Czech Republic, have also banned or restricted its distribution. Always check local regulations and laws.
9. Are there any documented incidents related to AH-7921 use?
AH-7921 has been associated with fatalities in different countries. These incidents have raised concerns about its safety and led to its legal restrictions.
10. Is AH-7921 still available for purchase online as a “research chemical”?
As of the last available information, AH-7921 had been sold on the internet as a “research chemical.” However, the legal status and availability may have changed, so it’s essential to verify current regulations and restrictions.
References
Brittain RT, Kellett DN, Neat ML, Stables R, conducted a study on anti-nociceptive effects in N-substituted cyclohexylmethylbenzamides, demonstrating potential pharmacological effects. (September 1973)- Hayes AG and Tyers MB determined the receptors mediating opiate side effects in mice, contributing to our understanding of opioid-related effects. (July 1983)
- Harper NJ, Veitch GB, and Wibberley DG explored 1-(3,4-Dichlorobenzamidomethyl)cyclohexyldimethylamine and related compounds as potential analgesics. (November 1974)
- A US patent was issued for “1-(3,4-Dichlorobenzamidomethyl)-cyclohexyldimethylamine” in 1976, assigned to Allen & Hanburys.
- In 2016, AH-7921 was officially placed into Schedule I in the United States, designating it as a controlled substance.
- Katselou M and colleagues expanded the list of new psychoactive opioids by studying AH-7921. (2015)
- Zawilska JB discussed AH-7921 as part of the expanding world of novel psychoactive substances, particularly opioids. (2017)
- Wohlfarth A and associates conducted in vitro metabolic stability assessments and metabolite identification of AH-7921, furthering our understanding of its pharmacokinetics. (August 2016)
- AH-7921 is cataloged as “3,4-dichloro-N-[[1-(dimethylamino)cyclohexyl]methyl]benzamide” in the PubChem database.
- Uchiyama N and colleagues detected AH-7921 in illegal products, highlighting its presence as a new psychoactive substance. (July 2013)
- In 2015, two horses tested positive for AH-7921, indicating its potential use in non-human contexts. (November 2015)
- Vorce SP and colleagues reported a fatality involving AH-7921, drawing attention to the substance’s dangers. (May 2014)
- Fels H and others discussed fatalities associated with synthetic opioids, including AH-7921, raising concerns about its safety. (August 2017)
- A review of previously published reports on AH-7921 was conducted in July 2018, summarizing existing knowledge about the substance.
- In Australia, AH-7921 was initially scheduled as a Prohibited Substance in May 2014. While the amendment was repealed in June 2014, it may still be considered a banned import.
- Israel banned the distribution of AH-7921 in December 2013, making it illegal.
- The United Kingdom included AH-7921 as a Class A drug in January 2015, under The Misuse of Drugs Act 1971 (Amendment) (No. 2) Order 2014.
- Brazil classified AH-7921 as an illegal drug in May 2015.
- China designated AH-7921 as a controlled substance as of October 2015.
- AH-7921 was banned in the Czech Republic as of a regulatory change.
- In the United States, AH-7921 was placed into Schedule I of the Controlled Substances Act on May 16, 2016, due to its lack of medical use.
- Canada classified AH-7921 as a Schedule I substance in the Controlled Drugs and Substances Act in 2016. Possession without legal authority can result in a maximum 7-year imprisonment.