Summary
AM-1220 is recognized for its potent and moderately selective agonistic effects on the cannabinoid receptor CB1, demonstrating approximately 19-fold selectivity for CB1 compared to the related CB2 receptor. This compound initially emerged in the early 1990s as a creation by a team led by Thomas D’Ambra at Sterling Winthrop. In the subsequent years, AM-1220 garnered significant attention from various researchers, notably the group under Alexandros Makriyannis at the University of Connecticut. It’s important to note that the (piperidine-2-yl)methyl side chain of AM-1220 possesses a stereocenter, leading to two enantiomers with notably distinct potencies. The (R)-enantiomer exhibits a remarkable Ki of 0.27 nM at CB1, while the (S)-enantiomer displays considerably weaker activity with a Ki of 217 nM.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 137642-54-7 (racemic) 134959-64-1 ((R)-enantiomer) |
|---|---|
| PubChem CID | 9929889 |
| ChemSpider | 26231035 |
| UNII | 05850M72P2(R)-enantiomer: 44NQ9RBE1B |
| CompTox Dashboard (EPA) | DTXSID50433001 |
| Chemical and physical data | |
| Formula | C26H26N2O |
| Molar mass | 382.507 g·mol−1 |
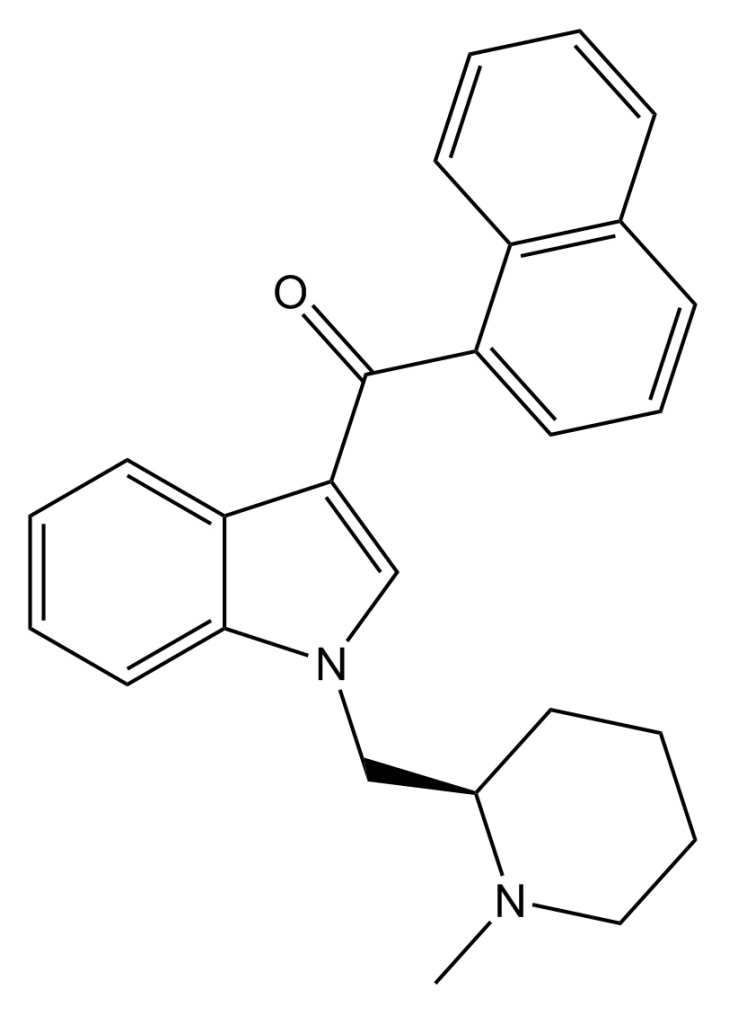
Related compounds
Numerous analogous compounds exhibit potent cannabinoid activity, featuring variations like indole ring substitution at the 2- or 6-positions, naphthyl ring substitution at the 4-position or replacement with substituted benzoyl rings or other moieties, and diverse groups replacing the 1-(N-methylpiperidin-2-ylmethyl) group, such as N-methylpyrrolidin-2-ylmethyl or N-methylmorpholin-3-ylmethyl. AM-1220 first emerged as a component in synthetic cannabis smoking blends in 2010.
Legal Status
United States:
In the United States, all CB1 receptor agonists of the 3-(1-naphthyl)indole class, including AM-1220, are classified as Schedule I Controlled Substances under the Controlled Substances Act.[8]
United Kingdom:
It’s illegal to supply, smuggle, distribute, transport, sell, or trade this pharmaceutical drug under the Psychoactive Substances Act 2016, which came into effect on May 26, 2016.
China:
As of October 2015, AM-1220 is considered a controlled substance in China.
FAQ
- What is AM-1220?
- AM-1220 is a synthetic drug that functions as a potent and moderately selective agonist for the cannabinoid receptor CB1. It exhibits around 19 times selectivity for CB1 over the related CB2 receptor.
- Who discovered AM-1220?
- AM-1220 was initially invented in the early 1990s by a team led by Thomas D’Ambra at Sterling Winthrop but has been the subject of subsequent research by various scientific groups, including the team led by Alexandros Makriyannis at the University of Connecticut.
- Does AM-1220 have different enantiomers?
- Yes, the (piperidine-2-yl)methyl side chain of AM-1220 contains a stereocenter, resulting in two enantiomers. The (R)-enantiomer is significantly more potent, with a Ki of 0.27 nM at CB1, while the (S)-enantiomer has a much weaker Ki of 217 nM.
- Are there related compounds to AM-1220?
- Yes, there are numerous related compounds with similar potent cannabinoid activity, often characterized by structural modifications. These modifications include substitutions on the indole ring at the 2- or 6-positions, variations in the naphthoyl ring at the 4-position, or replacement with substituted benzoyl rings and other groups. Additionally, diverse groups are replacing the 1-(N-methylpiperidin-2-ylmethyl) moiety, such as N-methylpyrrolidin-2-ylmethyl or N-methylmorpholin-3-ylmethyl.
- What is the legal status of AM-1220?
- The legal status of AM-1220 varies by country. In the United States, it falls under Schedule I Controlled Substances. In the United Kingdom, it’s illegal to supply or trade AM-1220 due to the Psychoactive Substances Act 2016. In China, AM-1220 is a controlled substance as of October 2015.
References
- Makriyannis and Deng’s Patent (2001): Makriyannis A and Deng H were granted a patent (WO patent 200128557) in 2001 for “Cannabimimetic indole derivatives,” which included the structural blueprint for various synthetic cannabinoids.
- D’Ambra’s Patent (1991): The US patent 5068234, granted to D’Ambra TE and colleagues in 1991, discusses “3-arylcarbonyl-1-(C-attached-N-heteryl)-1H-indoles,” a foundational document in the early development of synthetic cannabinoids.
- D’Ambra’s Research (1996): In 1996, D’Ambra published a paper titled “C-Attached aminoalkylindoles: potent cannabinoid mimetics” in the journal Bioorganic & Medicinal Chemistry Letters, outlining the potency of these compounds as cannabinoid mimetics.
- Deng’s PhD Dissertation (2000): In 2000, Hongfeng Deng completed a PhD dissertation at the University of Connecticut titled “Design and synthesis of selective cannabinoid receptor ligands: Aminoalkylindole and other heterocyclic analogs,” contributing to the understanding of selective cannabinoid receptor ligands.
- Willis and Horti’s Work (2005): In 2005, Willis PG, Pavlova OA, Chefer SI, Vaupel DB, Mukhin AG, and Horti AG conducted research on a novel series of aminoalkylindoles with potential for imaging the neuronal cannabinoid receptor by positron emission tomography.
- Makriyannis’ Later Patent (2010): In 2010, Makriyannis A and colleagues were granted another patent (US patent 7820144) titled “Receptor selective cannabimimetic aminoalkylindoles,” focusing on receptor-selective synthetic cannabinoids.
- Identification Chart (2010): In 2010, the “Head Shop ‘Legal Highs’ Active Constituents Identification Chart” likely played a role in identifying various active constituents in legal highs and synthetic cannabinoids.
- US Controlled Substances Act (Section 812): The United States’ Controlled Substances Act, Section 812, defines the schedules of controlled substances, including those that encompass synthetic cannabinoids.
- Chinese Regulation (2015): As of September 27, 2015, the “关于印发《非药用类麻醉药品和精神药品列管办法》的通知” by the China Food and Drug Administration outlines the regulation of non-medicinal narcotic drugs and psychotropic substances in China. This regulation had an impact on controlling substances like synthetic cannabinoids.