Summary
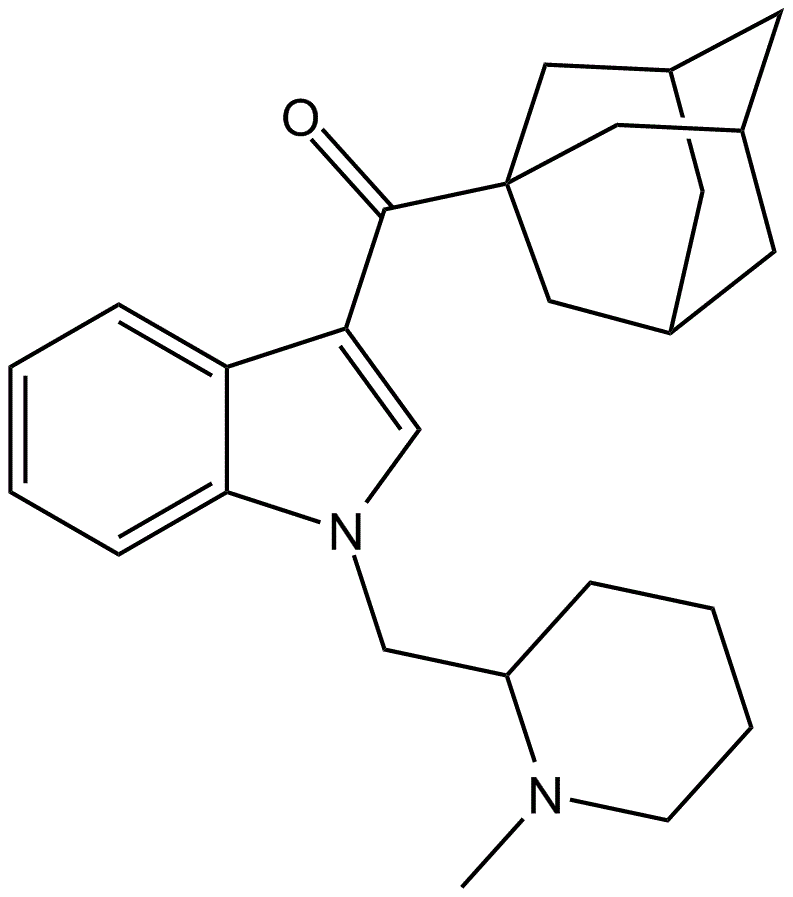
AM-1248 is a compound with disputed potency and selectivity, acting as a moderately potent agonist for both cannabinoid receptors CB1 and CB2. When the typical 3-(1-naphthyl) group found in many indole-derived cannabinoid ligands is replaced with an adamantyl group, it often imparts significant CB2 selectivity. However, by incorporating an N-methylpiperidin-2-ylmethyl substitution at the indole 1-position, it is possible to maintain reasonable CB1 affinity and selectivity. Notably, a related compound, 1-pentyl-3-(1-adamantyl)indole, was discovered being sold as a synthetic cannabinoid designer drug in Hungary in 2011, along with another synthetic cannabinoid, AM-679.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 335160-66-2 |
|---|---|
| PubChem CID | 10293794 |
| ChemSpider | 8469262 |
| UNII | 5EX9HEF4HK |
| Chemical and physical data | |
| Formula | C26H34N2O |
| Molar mass | 390.571 g·mol−1 |
Legality
On June 1, 2015, Sweden’s public health agency recommended categorizing AM-1248 as hazardous.
By October 2015, AM-1248 had been designated as a controlled substance in China.
FAQ
- What is AM-1248?
- AM-1248 is a synthetic compound with properties that make it an agonist for both cannabinoid receptors, CB1 and CB2. It is a part of the aminoalkylindole family of substances.
- What are the effects of AM-1248?
- AM-1248 is known for its agonistic activity at the CB1 and CB2 cannabinoid receptors. These receptors play roles in various physiological processes and are primarily associated with the endocannabinoid system. The effects of AM-1248 may include alterations in mood, perception, and pain perception.
- Is AM-1248 legal?
- The legal status of AM-1248 varies by country. It is essential to check the specific regulations in your region, as some countries have banned or classified it as a controlled substance due to its potential for misuse.
- Is AM-1248 safe for consumption?
- The safety of AM-1248 for human consumption is not well-documented, and it is not intended for such use. It is a synthetic research compound and should only be handled by qualified individuals in controlled laboratories.
- How is AM-1248 used in research?
- AM-1248 is primarily used in scientific research to understand the functioning of the endocannabinoid system and to investigate the potential therapeutic applications of cannabinoid agonists.
- Are there any known health risks associated with AM-1248?
- AM-1248 may have potential health risks, including adverse effects on mood, cognition, and physical health. Its misuse can lead to unpredictable and potentially harmful outcomes. Researchers and individuals should exercise caution and adhere to relevant safety guidelines when handling this compound.
- Where can I find more information about AM-1248?
- To access more information about AM-1248, you can refer to scientific literature, research publications, and relevant government agency websites that regulate controlled substances. Always adhere to local laws and regulations when seeking information about or working with AM-1248.
References
- A study by Frost JM, Dart MJ, and colleagues in January 2010 explored the effects of N1-substituted indole side chain variations on the activity of CB2 cannabinoid receptors. The research delved into indol-3-ylcycloalkyl ketones and their potential interactions with the CB2 receptor. (Source: Journal of Medicinal Chemistry, PMID 19921781)
- In 2010, a US patent (No. 7820144) was granted to Makriyannis A and Deng H for “Receptor-selective cannabimimetic aminoalkylindoles.” This patent likely covers novel compounds with specific receptor affinity. (Source: US Patent Office)
- A WO (World Intellectual Property Organization) patent (No. 200128557) titled “Cannabimimetic indole derivatives” was granted on June 7, 2001, to Makriyannis A and Deng H. This patent may encompass various indole derivatives with cannabimimetic properties. (Source: World Intellectual Property Organization)
- A study conducted by Jankovics P, Váradi A, Tölgyesi L, and others in January 2012 focused on the “Detection and identification of the new potential synthetic cannabinoids 1-pentyl-3-(2-iodobenzoyl)indole and 1-pentyl-3-(1-adamantoyl)indole in seized bulk powders in Hungary.” The research aimed to identify and characterize these emerging synthetic cannabinoids. (Source: Forensic Science International, PMID 21813254)
- Sweden made recommendations to classify AM-1248 as a hazardous substance on June 1, 2015. This suggests that there were concerns regarding its safety or potential risks. (Source: Swedish Public Health Agency)
- As of October 2015, AM-1248 was designated as a controlled substance in China. This indicates that it is subject to strict regulations in the country, likely due to its potential for misuse. (Source: China Food and Drug Administration)