Summary
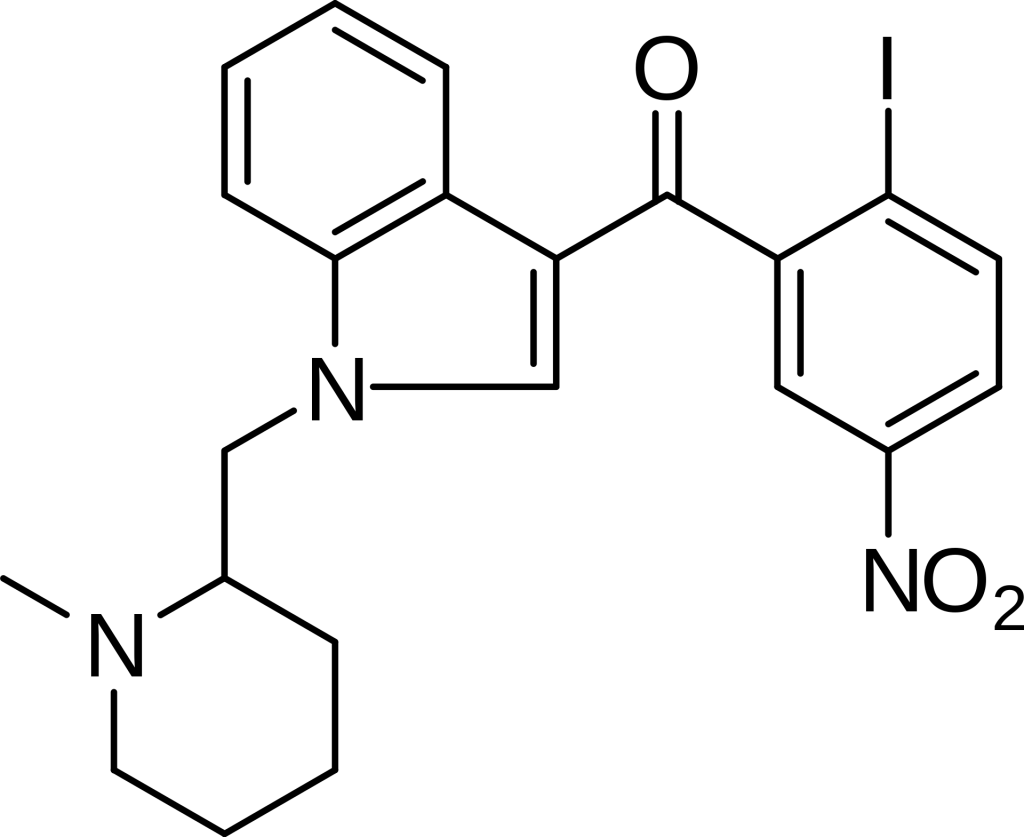
AM-1241, also known as 1-(methylpiperidin-2-ylmethyl)-3-(2-iodo-5-nitrobenzoyl)indole, is a member of the aminoalkylindole family, recognized for its role as a potent and highly selective agonist for the cannabinoid receptor CB2. With a remarkable Ki value of 3.4 nM at CB2, it exhibits substantial selectivity, being approximately 80 times more selective for CB2 compared to the related CB1 receptor.
Notably, AM-1241 has demonstrated analgesic properties in animal studies, particularly in “atypical” pain conditions such as hyperalgesia and allodynia. These effects are believed to be mediated through CB2 receptors, resulting in the peripheral release of endogenous opioid peptides. Additionally, AM-1241 is known to activate the TRPA1 channel directly, contributing to its analgesic mechanisms.
Furthermore, AM-1241 has shown promise in treating amyotrophic lateral sclerosis (ALS) in animal models, emphasizing its potential therapeutic applications beyond pain management.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 444912-48-5 |
|---|---|
| PubChem CID | 10141893 |
| IUPHAR/BPS | 3316 |
| ChemSpider | 8317404 |
| UNII | DLM851L3RD |
| ChEMBL | ChEMBL408430 |
| ECHA InfoCard | 100.164.689 |
| Chemical and physical data | |
| Formula | C22H22IN3O3 |
| Molar mass | 503.340 g·mol−1 |
Effects in bone cancer model
Researchers explored the potential antihyperalgesic properties of AM-1241 in a murine model of bone cancer. In this study, sarcoma cells were introduced into a mouse’s femur, followed by twice-daily injections of AM-1241. The results indicated that treatment with AM-1241 effectively alleviated both spontaneous and evoked pain while also mitigating bone loss and the risk of tumor-induced fractures. Interestingly, pre-administration of the CB2 antagonist SR-144,528 reversed the immediate effects of AM-1241 on both spontaneous and evoked pain, with no impact observed when SR-144,528 was administered alone.
FAQ
- What is AM-1241, and how does it relate to cannabinoid receptors?
- AM-1241 is a chemical compound belonging to the aminoalkylindole family. It acts as a potent and highly selective agonist for the cannabinoid receptor CB2.
- What is the binding affinity of AM-1241 for CB2 and CB1 receptors?
- AM-1241 has a remarkable Ki (binding affinity) of 3.4 nM at CB2, demonstrating approximately 80 times selectivity for CB2 over the related CB1 receptor.
- What are the analgesic effects of AM-1241, and how does it work in pain management?
- AM-1241 has shown analgesic properties, particularly in “atypical” pain conditions like hyperalgesia and allodynia. These effects are believed to be mediated through CB2 receptors, which result in the peripheral release of endogenous opioid peptides. Additionally, AM-1241 directly activates the TRPA1 channel, contributing to its analgesic mechanisms.
- Has AM-1241 shown promise in other medical applications?
- Yes, AM-1241 has demonstrated potential in treating amyotrophic lateral sclerosis (ALS) in animal models, suggesting its broader therapeutic applications beyond pain management.
- How was the antihyperalgesic effect of AM-1241 studied in a bone cancer model?
- In a murine bone cancer model, sarcoma cells were injected into the femur of a mouse, followed by twice-daily injections of AM-1241. The research found that AM-1241 effectively reduced both spontaneous and evoked pain, as well as mitigated bone loss and the risk of tumour-induced fractures.
- What is the role of the CB2 antagonist SR-144,528 about AM-1241?
- Pretreatment with the CB2 antagonist SR-144,528 reversed the immediate effects of AM-1241 on both spontaneous and evoked pain, suggesting the involvement of CB2 receptors in AM-1241’s analgesic properties.
- Is AM-1241 available for medical use or research purposes?
- AM-1241 is primarily used for research purposes. Researchers should ensure compliance with local and national regulations when working with such compounds. It is not approved for medical use in humans.
- Where can I find more information about AM-1241 and related research?
- To learn more about AM-1241 and its applications in cannabinoid research and pain management, you can explore scientific journals academic databases, and consult experts in the field of cannabinoid pharmacology. Always adhere to appropriate safety and legal guidelines when researching or handling such substances.
References
- In a study by Yao BB, Mukherjee S, and colleagues (September 2006), the pharmacological characterization of AM1241, a protean agonist at the cannabinoid CB2 receptor, was explored. The research, published in the “British Journal of Pharmacology” (149(2), 145–154), delved into the multifaceted actions of AM1241 at CB2 receptors, shedding light on its pharmacological profile.
- Bingham B, Jones PG, Uveges AJ, and others (August 2007) conducted research into the species-specific pharmacological effects of the CB2 selective ligand AM1241 and its resolved enantiomers. Their findings, published in the “British Journal of Pharmacology” (151(7), 1061–1070), highlighted the differential responses to AM1241 and its enantiomers in various species, offering valuable insights into its pharmacological effects.
- Ibrahim MM, Deng H, Zvonok A, and colleagues (September 2003) investigated the inhibition of experimental neuropathic pain through the activation of CB2 cannabinoid receptors by AM1241. This research, featured in the “Proceedings of the National Academy of Sciences of the United States of America” (100(18), 10529–10533), explored the role of CB2 receptors in pain modulation, particularly in the peripheral nervous system.
- Marriott KS and Huffman JW (2008) provided insights into recent advances in the development of selective ligands for the cannabinoid CB(2) receptor. Their review, published in “Current Topics in Medicinal Chemistry” (8(3), 187–204), highlighted the progress in designing compounds targeting CB2 receptors for various applications.
- Beltramo M, Bernardini N, Bertorelli R, and colleagues (March 2006) discussed CB2 receptor-mediated antihyperalgesia and its potential involvement in neural mechanisms. Their research in “The European Journal of Neuroscience” (23(6), 1530–1538) delved into the neural pathways through which CB2 receptor activation may alleviate hyperalgesia.
- Ibrahim MM, Porreca F, Lai J, and others (February 2005) explored how CB2 cannabinoid receptor activation produces antinociception by stimulating the peripheral release of endogenous opioids. This study, published in the “Proceedings of the National Academy of Sciences of the United States of America” (102(8), 3093–3098), unveiled a mechanism involving endogenous opioids in CB2-mediated pain relief.
- Akopian AN, Ruparel NB, Patwardhan A, and colleagues (January 2008) investigated the desensitization of sensory neurons to capsaicin and mustard oil responses via TRPA1 activation by cannabinoids. Their work, featured in “The Journal of Neuroscience” (28(5), 1064–1075), provided insights into the interaction between cannabinoids and TRPA1 receptors in sensory neurons.
- Kim K, Moore DH, Makriyannis A, and Abood ME (August 2006) studied the effects of AM1241, a CB2 receptor selective compound, in delaying disease progression in a mouse model of amyotrophic lateral sclerosis (ALS). Their research, published in the “European Journal of Pharmacology” (542(1–3), 100–105), highlighted potential therapeutic applications for AM1241 in ALS.
- Shoemaker JL, Seely KA, Reed RL, and colleagues (April 2007) found that the CB2 cannabinoid agonist AM-1241 prolonged survival in a transgenic mouse model of ALS when initiated at symptom onset. Their research, published in the “Journal of Neurochemistry” (101(1), 87–98), demonstrated the potential for AM-1241 in extending the survival of ALS-affected mice.
- Lozano-Ondoua AN, Wright C, Vardanyan A, and others (April 2010) explored the attenuation of bone cancer-induced pain and bone loss through the use of a cannabinoid 2 receptor agonist. Their findings, featured in “Life Sciences” (86(17–18), 646–653), highlighted the potential of CB2 agonists in managing bone cancer-related pain and bone loss.