Summary
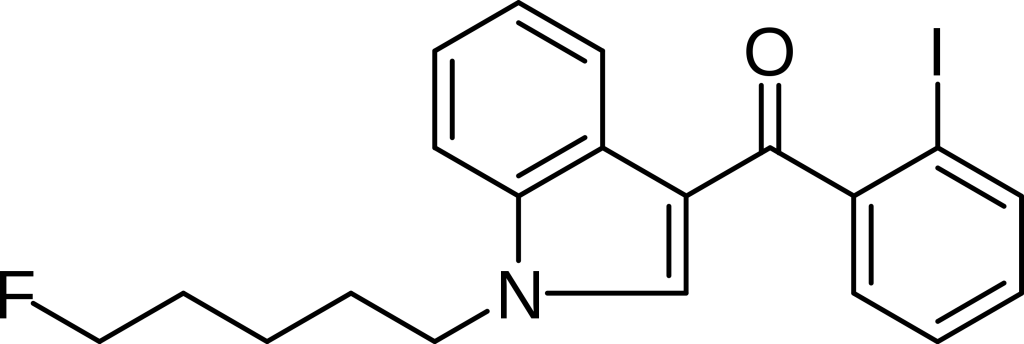
AM-694, known chemically as 1-(5-fluorophenyl)-3-(2-iodobenzoyl)indole, functions as a designer drug with a specific role as a potent and selective agonist for the cannabinoid receptor CB1. This compound is frequently utilized in scientific research to map the distribution of CB1 receptors within the body.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 335161-03-0 |
|---|---|
| PubChem CID | 9889172 |
| ChemSpider | 8064843 |
| UNII | 6RK7KN7L1O |
| ChEBI | CHEBI:138017 |
| CompTox Dashboard (EPA) | DTXSID30187156 |
| Chemical and physical data | |
| Formula | C20H19FINO |
| Molar mass | 435.281 g·mol−1 |
Pharmacology
AM-694 acts as an agonist for cannabinoid receptors, demonstrating remarkable binding affinity. Specifically, it boasts a Ki of 0.08 nM at CB1 receptors and exhibits a notable 18-fold selectivity over CB2 receptors, with a Ki of 1.44 nM. The precise mechanism responsible for this exceptionally high CB1 binding affinity remains unclear. However, this distinctive characteristic renders the 18F radiolabeled derivative of AM-694 invaluable for mapping the distribution of CB1 receptors throughout the body.
Metabolism
Regarding its metabolism, AM-694 undergoes various pathways, including hydrolytic defluorination, carboxylation, and monohydroxylation of the N-alkyl chain. These metabolic processes contribute to understanding how the body processes this compound.
FAQ
- What is AM-694, and what does it play in cannabinoid research?
- AM-694 is a compound known for its function as an agonist for cannabinoid receptors. It is frequently utilized in scientific research to study and map the distribution of CB1 receptors in the body.
- What is the binding affinity of AM-694 for CB1 and CB2 receptors?
- AM-694 exhibits exceptional binding affinity, with a Ki (binding constant) of 0.08 nM at CB1 receptors and a remarkable 18-fold selectivity over CB2 receptors, possessing a Ki of 1.44 nM.
- Why is AM-694’s high CB1 binding affinity significant?
- The precise mechanism behind AM-694’s unusually high CB1 binding affinity remains unclear. However, this unique characteristic is valuable, as it enables the development of an 18F radiolabeled derivative of AM-694, which is used for mapping the distribution of CB1 receptors within the body.
- What can researchers learn from mapping CB1 receptor distribution using AM-694?
- Mapping CB1 receptor distribution with AM-694 is essential for understanding the localization of these receptors in the body. This knowledge can provide insights into the physiological and neurological functions associated with CB1 receptors.
- How is AM-694 metabolized in the body?
- AM-694 undergoes several metabolic pathways, including hydrolytic defluorination, carboxylation, and monohydroxylation of the N-alkyl chain. These metabolic processes offer insights into how the body processes and eliminates this compound.
- Is AM-694 legal for use in research or recreational purposes?
- The legality of AM-694 can vary by jurisdiction. Typically, it is used for research purposes, and researchers should ensure compliance with local and national regulations when working with such compounds.
- Where can I find more information about AM-694 and its applications in research?
- To learn more about AM-694 and its role in cannabinoid research, you can explore scientific journals and academic databases and seek guidance from experts in cannabinoid pharmacology. Always adhere to appropriate safety and legal guidelines when researching or handling such substances.
References
- In a study by Willis PG, Katoch-Rouse R, and Horti AG (August 2003), researchers focused on the regioselective F‐18 radiolabeling of AM694, a ligand for the CB1 cannabinoid receptor. Their findings, published in the “Journal of Labelled Compounds and Radiopharmaceuticals” (46(9), 799–804), shed light on the radiolabeling techniques used in cannabinoid receptor research.
- WO patent 200128557, granted to Makriyannis A and Deng H on June 7, 2001, represents a pivotal patent in the field of cannabinoid research. Titled “Cannabimimetic Indole Derivatives,” this patent underscores the development of indole derivatives with cannabimimetic properties, paving the way for further research and innovation.
- Grigoryev A, Kavanagh P, and Melnik A (February 2013) conducted research focused on the detection of urinary metabolites of AM-694, a high-affinity cannabimimetic compound. Their work, published in “Drug Testing and Analysis” (5(2), 110–115), explored the methods of identifying metabolites associated with this synthetic cannabinoid.
- Apirakkan O, Gavrilović I, Cowan DA, and Abbate V (July 2020) delved into the in vitro phase I metabolic profiling of several synthetic cannabinoids, including AM-694. Their research, featured in “Chemical Research in Toxicology” (33(7), 1653–1664), contributed to our understanding of the metabolic pathways and profiles of these compounds, offering insights into their effects on the body.