Contents
Summary
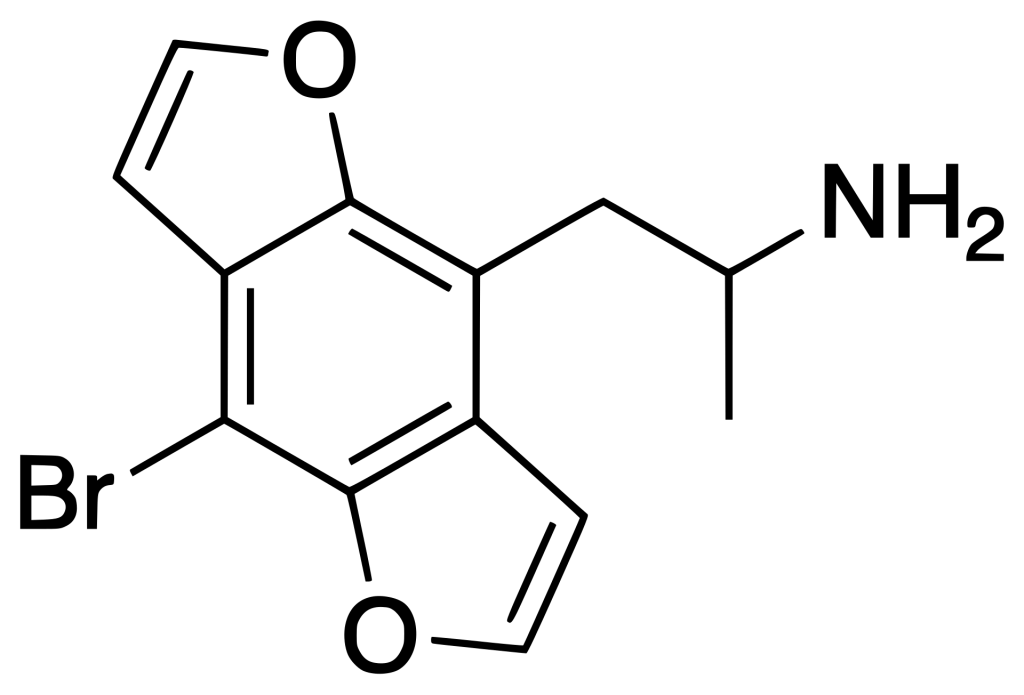
Bromo-DragonFLY, also known as 3C-Bromo-Dragonfly or DOB-Dragonfly, belongs to the phenethylamine family of substances. It functions as a powerful full agonist for the 5-HT2A receptor.
| Identifiers | |
|---|---|
| CAS Number | 502759-67-3 |
| 3D model (JSmol) | Interactive imageInteractive image |
| ChEMBL | ChEMBL149024 |
| ChemSpider | 8014776 8719838 R 8672089 S |
| PubChemCID | 983905710544447 R10496688 S |
| UNII | GC9M7R36OI |
History
In 1998, Matthew Parker synthesized Bromo-DragonFLY in the laboratory of David E. Nichols. Similar to the preceding and less potent dihydrofuran series of compounds dubbed FLY, the vocabulary of Bromo-DragonFLY was inspired by its surface-level structural resemblance to a dragonfly.
Pharmacology
Bromo-DragonFLY exhibits robust binding affinity at the 5-HT2A receptor (Ki 0.04 nM), demonstrating a comparable strong affinity at the 5-HT2C receptor (Ki 0.02 nM), and displaying moderate affinity for the 5-HT2B receptor (Ki 0.19 nM). Additionally, it functions as an MAO-A inhibitor, which leads to the potent inhibition of the oxidative deamination of 5-HT, thereby elevating its risk profile.
Chemistry
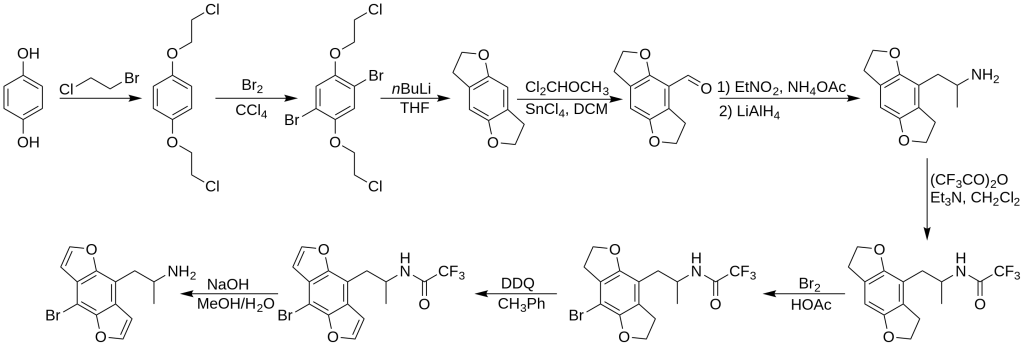
The initial synthesis of racemic Bromo-DragonFLY was documented by David E. Nichols in 1998, building upon prior research concerning the tetrahydrobenzodifuran analog of DOB. In this process, hydroquinone served as the starting material, undergoing dialkylation with 1-bromo-2-chloroethane, followed by bromination and treatment with n-butyllithium to yield the tetrahydrobenzodifuran ring system. Subsequent steps involved formylation of the ring system, leading to the generation of the nitro propene derivative via condensation with nitroethane under ammonium acetate catalysis. Lithium aluminum hydride reduction produced the amine intermediate, protected with trifluoroacetic anhydride. Further para-bromination with elemental bromine and tetrahydrobenzodifuran ring system oxidation using DDQ followed, culminating in the removal of the trifluoroacetyl protecting group to obtain racemic Bromo-DragonFLY in the form of the R and S enantiomers.
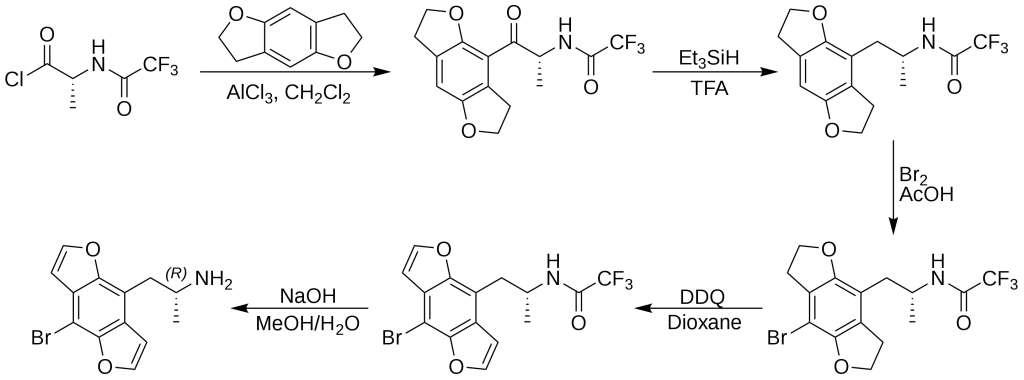
In 2001, David E. Nichols presented an enantiospecific synthesis method for Bromo-DragonFLY, allowing for the individual study of the R and S enantiomers. Subsequent investigations revealed that (R)-(-)-Bromo-DragonFLY exhibited superior binding affinity at the 5-HT2A and 5-HT2C receptors compared to (S)-(-)-Bromo-DragonFLY. The synthesis of the more active R enantiomer involved the reaction of a derivative of D-alanine with 2,3,6,7-tetrahydrobenzodifuran through a Friedel–Crafts acylation, resulting in an intermediate containing a β-keto moiety that was eliminated through treatment with triethylsilane in trifluoroacetic acid. The process continued with para-bromination and DDQ-induced oxidation of the ring system, leading to the deprotection of the amine and the production of (R)-(-)-Bromo-DragonFLY.
Dosage
Information regarding the toxicological implications and appropriate dosage of Bromo-DragonFLY remains limited as no significant data is derived from human consumption. Nonetheless, this compound’s generally reported recreational dosage falls within the range of 500 to 1000 μg. It is essential to note that fatalities have been documented at blood concentrations of 0.69 mg/mL, roughly equivalent to 700 μg of Bromo-DragonFLY.
Toxicity
The potential toxicity associated with Bromo-DragonFLY in humans is considered notably high, evident from reported cases of at least five fatalities linked to its consumption in Norway, Sweden, Denmark, Finland, and the United States. An alarming incident in October 2009 revealed the mislabeling of a batch of Bromo-DragonFLY as the less potent compound 2C-B-FLY, leading to several lethal overdoses and subsequent hospitalizations. The implicated batch contained significant synthesis impurities, potentially contributing to its heightened toxicity.
Notably, severe overdoses of Bromo-DragonFLY have resulted in vasoconstrictive actions leading to tissue necrosis, as observed in a 2007 case where a Swedish male suffered extensive limb damage, requiring amputation due to the compound’s long-acting vasoconstrictor effects. Although limited treatment efficacy was reported, tolazoline has shown promise as an effective intervention in such cases.
Additionally, cases involving disturbing experiences and health complications linked to overdose have been documented, including instances of inhalation of vomit leading to near-fatal asphyxia and occurrences of seizures. Tragic incidents, such as the one in Copenhagen in 2009 and another in the United States in 2011, highlight the detrimental consequences of mistaken dosing, resulting in terrifying hallucinations, seizures, and fatalities.
Drug prohibition laws
- United States:
- Bromo-DragonFLY remains unscheduled at the federal level in the United States. However, its sale for human consumption could lead to prosecution. Notably, it is categorized as a Schedule I substance in Oklahoma.
- Canada:
- According to the Canadian Controlled Drugs and Substances Act, Bromo-DragonFLY is classified under Schedule III. The Act broadly encompasses “2C-phenethylamines and their salts, derivatives, isomers, and salts of derivatives and isomers,” including compounds with a 2,5-dimethoxyphenethylamine core, such as the 2C family, the DOx chemical class, the TMA family, Aleph, NBOMe, the 25x-NBx series, and Bromo-DragonFLY itself.
- United Kingdom:
- Bromo-DragonFLY has garnered media attention as a Class A drug in the UK. However, its status under the UK phenylethylamine catch-all clause remains unclear, with discussions highlighting structural similarities and differences to the phenylethylamine class. While it is not explicitly named in the Misuse of Drugs Act, it would fall under the UK Psychoactive Substances Act 2016 if sold or traded for human consumption.
- Sweden:
- In Sweden, Bromo-Dragonfly has been added to schedule IV under the “substances, plant materials, and fungi without medical use” category. It was declared a “health hazard” and is prohibited for sale, purchase, retail, or possession as of July 15, 2007.
- Denmark:
- The Danish government banned Bromo-DragonFLY on December 3, 2007, categorizing it as a dangerous narcotic. Any involvement in its possession, manufacture, importation, supply, or usage can lead to legal action.
- Norway:
- Bromo-DragonFLY is currently listed on the Norwegian narcotics roster.
- Poland:
- Presently, Bromo-DragonFLY is an uncontrolled substance in Poland.
- Romania:
- Under Law 143/2000, Bromo-DragonFLY has been declared an illegal substance in Romania since February 10, 2010.
- Australia:
- In Australia, Bromo-DragonFLY was included in Schedule 2 of the Queensland Drugs Misuse Regulation 1987 as of September 9, 2011. It is also listed under Schedule 9 (Prohibited) of the Poisons Standard nationwide.
- Finland:
- As of March 12, 2012, Bromo-DragonFLY is considered an illegal designer drug in Finland.
FAQ
1. What is Bromo-DragonFLY?
- Bromo-DragonFLY is a powerful psychoactive substance in the phenethylamine class known for its hallucinogenic effects and potent binding affinity to serotonin receptors.
2. What are the reported effects of Bromo-DragonFLY?
- The effects of Bromo-DragonFLY include hallucinations, altered sensory perception, and vasoconstriction, potentially leading to severe health complications and even fatalities in some cases.
3. What is the legal status of Bromo-DragonFLY in different countries?
- Bromo-DragonFLY’s legal status varies across countries. While it remains unscheduled at the federal level in the United States, it is classified as a controlled substance in several countries, including Canada, the United Kingdom, Sweden, Denmark, Norway, Australia, and others.
4. What are the potential health risks associated with Bromo-DragonFLY?
- Bromo-DragonFLY poses significant health risks, including vasoconstriction leading to tissue necrosis, seizures, and even fatalities at high doses. Overdose can result in life-threatening complications.
5. Is Bromo-DragonFLY available for medicinal purposes?
- In most jurisdictions, bromo-DragonFLY is not approved for medical use and is considered a dangerous and illicit substance. Medical professionals do not prescribe it.
6. How should one approach the use of Bromo-DragonFLY?
- The use of Bromo-DragonFLY is highly discouraged due to its potent and unpredictable effects, along with the associated health risks. Individuals are advised to avoid using this substance for recreational or other purposes.
7. What should be done in case of a suspected Bromo-DragonFLY overdose?
- In case of a suspected Bromo-DragonFLY overdose, immediate medical attention should be sought. Prompt intervention is crucial in managing this substance’s potentially life-threatening symptoms and complications.
8. Are there any known antidotes or treatments for Bromo-DragonFLY intoxication?
- While there is no specific antidote for Bromo-DragonFLY intoxication, certain interventions may be employed in medical settings to manage symptoms and provide supportive care. However, the efficacy of treatments may vary based on individual cases.
9. What measures can be taken to prevent Bromo-DragonFLY-related harm?
- Preventive measures include raising awareness about the risks associated with Bromo-DragonFLY, implementing strict regulatory controls, and promoting education about the dangers of its use and the legal consequences of its possession and distribution.
10. Where can I find more information on Bromo-DragonFLY?
– For further information on Bromo-DragonFLY, it is advisable to consult reputable scientific sources, drug enforcement agencies, and healthcare professionals. Additionally, information from published research papers and official government resources can provide valuable insights into the properties and risks associated with this substance.
References
- Parker, MJ; Marona-Lewicka, D; Lucaites, VL; Nelson, DL; Nichols, DE. (December 1998). “A distinctive (benzodifuranyl)aminoalkane displaying highly potent activity at the 5-HT2A receptor”. Journal of Medicinal Chemistry. 41 (26): 5148–5149. doi:10.1021/jm9803525. PMID 9857084.
- James, J. Chambers. (2001). “Enantiospecific construction and pharmacological assessment of a set of extraordinarily potent, conformationally constrained 5-HT2A/2C receptor agonists”. Journal of Medicinal Chemistry. 44 (6): 1003–1010. doi:10.1021/jm000491y. PMID 11300881.
- “Bromo-dragonfly – Dangerous Abused Drug”. The perils of substance misuse are evident with Bromo-dragonfly. Per, aged 35, faced mutilation due to the lethal drug – Required ten days of respiratory care after consuming Bromo-Dragonfly.