Summary
Dimethoxybromoamphetamine (DOB), alternatively referred to as brolamfetamine (INN), represents a psychedelic substance and substituted amphetamine belonging to the phenethylamine class of compounds. The synthesis of DOB was initially carried out by Alexander Shulgin in 1967, with comprehensive details regarding its production and effects extensively documented in Shulgin’s renowned publication “PiHKAL: A Chemical Love Story.”
| Identifiers | |
|---|---|
| CAS Number | 64638-07-9 (racemate) 43061-15-0 (R) 43061-16-1 (S) |
| 3D model (JSmol) | Interactive image |
| ChEMBL | ChEMBL6607 |
| ChemSpider | 55902 |
| DrugBank | DB01484 |
| IUPHAR/BPS | 155 |
| PubChemCID | 62065 |
| UNII | 67WJC4Y2QY (racemate) C918IS60W6 (R) NXQ58PZ30E (S) |
| CompTox Dashboard(EPA) | DTXSID5050428 |
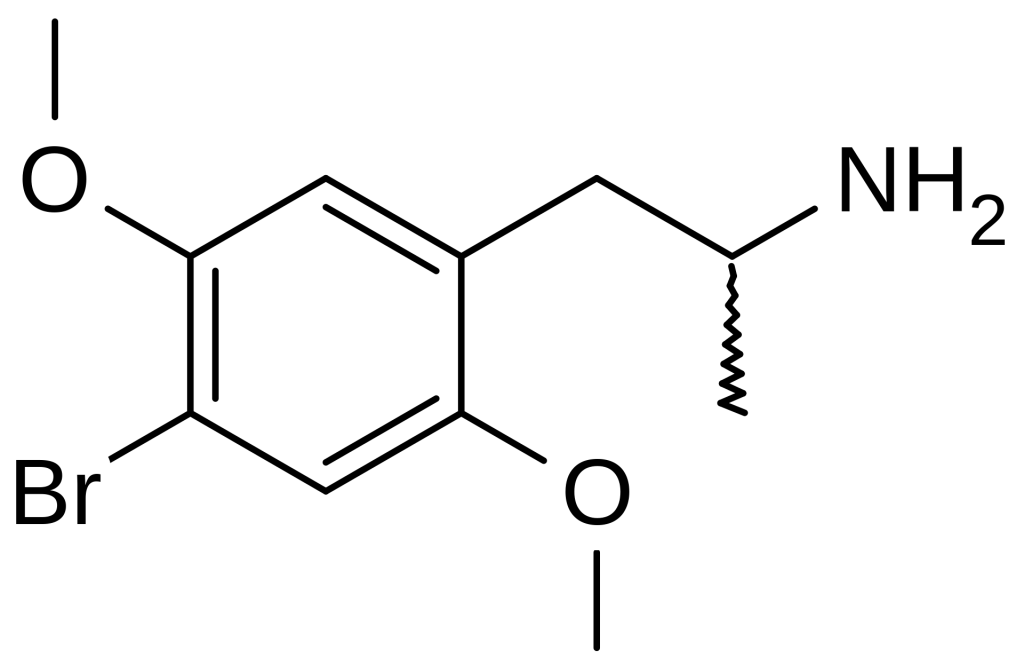
Chemistry
The chemical’s complete name is 2,5-dimethoxy-4-bromoamphetamine. DOB possesses a stereocenter, with the R-(–)-DOB being the eutomer. This discovery is significant as it implies that it interacts with different receptors than most other phenethylamines, such as MDMA, where the R-isomer functions as the distomer. The precise extent of DOB’s toxicity remains incompletely understood; however, it is known that elevated doses can induce severe vasoconstriction of the extremities. Within PiHKAL, DOB is recognized as one of the most potent compounds. While its active dose resembles DOI, another psychedelic amphetamine, DOB, has exhibited greater efficacy in triggering downstream effects mediated by 5-HT2 receptors, suggesting a slightly higher risk of overdose-induced vasoconstriction than DOI. Notably, the absence of the amphetamine-related α-methyl group leads to the formation of 2C-B, a compound with lower affinity for the 5-HT2A receptor and weaker receptor agonist properties, resulting in significantly reduced vasoconstriction.
Pharmacology
DOB functions as an agonist or partial agonist at the 5-HT2A, 5-HT2B, and 5-HT2C receptors. Its psychedelic impact primarily stems from its agonistic activity at the 5-HT2A receptor. Because of its specific receptor selectivity, DOB is frequently employed in scientific investigations concerning the 5-HT2 receptor subfamily. Additionally, it acts as an agonist of human TAAR1.
Studies suggest that DOB might function as a prodrug metabolized within the lungs. In excessive doses of this hallucinogenic substance, diffuse arterial spasms can occur. However, the vasospasm is responsive to intra-arterial and intravenous vasodilators like tolazoline.
Legal status
Internationally, DOB is classified as a Schedule I substance by the Convention on Psychotropic Substances. It is legally permitted solely for medical, industrial, or scientific purposes.
Canada:
In Canada, DOB is categorized as a Schedule 1 substance due to its analogical relation to amphetamine.
Australia:
Australia designates DOB as a Schedule 9 prohibited substance under the Poisons Standard (as of February 2017). This classification identifies it as a substance with potential for misuse or abuse, and its manufacture, possession, sale, or use is generally prohibited by law. Exceptions are made for medical or scientific research and analytical, educational, or training purposes with approval from relevant Commonwealth and State or Territory Health Authorities.
Russia:
In Russia, DOB is listed as a Schedule I substance, and possessing at least 10 mg is considered criminal.
United Kingdom:
In the United Kingdom, DOB is recognized as a Class A drug per the Misuse of Drugs Act 1971.
FAQ
1. What is 2,5-Dimethoxy-4-bromoamphetamine (DOB)?
- 2,5-Dimethoxy-4-bromoamphetamine, commonly known as DOB, is a potent psychedelic compound and substituted amphetamine, belonging to the phenethylamine class of substances.
2. What are the effects of DOB on the body and mind?
- DOB induces psychedelic experiences, altering sensory perception and producing hallucinatory effects, often accompanied by heightened sensory awareness and emotional shifts.
3. How is DOB regulated internationally?
- DOB is internationally recognized as a Schedule I substance under the Convention on Psychotropic Substances, indicating its restricted legal status for specific authorized purposes such as medical, industrial, or scientific use.
4. What legal status does DOB have in Canada, Australia, Russia, and the United Kingdom?
- In Canada, DOB is classified as a Schedule 1 substance. In Australia, it is categorized as a Schedule 9 prohibited substance. In Russia, it is labeled as a Schedule I substance, and in the United Kingdom, it is considered a Class A drug under the Misuse of Drugs Act 1971.
5. What are the potential risks associated with DOB usage?
- Consumption of DOB, especially at high doses, can lead to severe vasoconstriction and other potentially harmful physiological effects. It is essential to be aware of the risks involved in its use.
6. Can DOB be used for medicinal purposes?
- In many jurisdictions, DOB is not approved for medical use and is considered a controlled and potentially dangerous substance. Its use is strictly limited to authorized research and scientific studies.
7. What should one do in case of suspected DOB overdose or adverse reactions?
- If there is a suspected overdose or adverse reaction to DOB, seeking immediate medical assistance is crucial. Prompt intervention can help manage the effects and prevent any further complications.
8. Where can I find reliable information about DOB and its effects?
- Reputable scientific resources, drug enforcement agencies, and healthcare professionals can provide reliable information about DOB and its effects. Additionally, published research papers and official government sources offer valuable insights into the properties and risks associated with this substance.
9. What precautions should one take before considering a DOB?
- It is highly advisable to thoroughly understand the legal implications, potential health risks, and associated dangers of DOB usage. Considering the possible adverse effects, avoiding this substance for recreational or non-medical purposes is recommended.
10. Are there any recognized treatments for DOB-related complications or adverse effects?
- While there is no specific antidote for DOB-related complications, seeking professional medical assistance to manage symptoms and provide necessary support is crucial in mitigating any potential harm from its usage.
References
- Anvisa’s recent resolution, RDC Nº 804, delves into the regulatory control of specific substances, including 2,5-Dimethoxy-4-bromoamphetamine, emphasizing their narcotic and psychotropic nature. This resolution, published in the Diário Oficial da União in July 2023, contributes to the ongoing oversight and management of controlled substances within Brazil.
- The World Health Organization’s International Nonproprietary Names (INN) for Pharmaceutical Substances has acknowledged the pharmacological significance of 2,5-Dimethoxy-4-bromoamphetamine, documenting its properties and pharmaceutical relevance, thereby adding to the global understanding of this compound.
- Notable researchers, including Shulgin, Sargent, and Naranjo, explored the biochemical characteristics of 2,5-Dimethoxy-4-bromoamphetamine in a 1971 study published in Pharmacology, shedding light on its centrality as an active amphetamine analog with implications for central nervous system activity.
- The elucidation of differential phospholipase C activation by phenylalkylamine serotonin 5-HT2A receptor agonists, including 2,5-Dimethoxy-4-bromoamphetamine, has been a subject of investigation in the Journal of Neurochemistry, providing valuable insights into its mechanism of action and potential neurochemical impacts.
- Ray’s research on psychedelics and the human receptorome, published in PLOS ONE, offers a comprehensive understanding of the interplay between 2,5-Dimethoxy-4-bromoamphetamine and the human receptor system, highlighting its potential effects on various receptors and associated physiological responses.
- Lewin, Miller, and Gilmour’s contributions to the Binding Database shed light on the intricate binding interactions of 2,5-Dimethoxy-4-bromoamphetamine, enhancing our knowledge of its pharmacological profile and its potential effects on specific binding sites within the body.
- The remarkable insights provided by Shulgin’s exploration of potential prodrugs, including DOB, available through his “Ask Dr. Shulgin Online” platform, are instrumental in understanding the compound’s possible metabolic pathways and subsequent physiological effects.
- Clinical observations of diffuse vascular spasm associated with the ingestion of 4-Bromo-2,5-Dimethoxyamphetamine are reported in JAMA: The Journal of the American Medical Association, contributing to the understanding of its potential physiological implications and associated health risks.
- The categorization of 2,5-Dimethoxy-4-bromoamphetamine in the list of psychotropic substances under international control, archived for reference, emphasizes the compound’s regulated status and its significance within the context of international drug control measures.
- Legislative measures across different countries, such as the Poisons Standard in Australia and the resolution by the Russian government, underscore the global effort to monitor and control the production, distribution, and use of 2,5-Dimethoxy-4-bromoamphetamine, reflecting the ongoing international concern regarding its potential impact and misuse.