Contents
Summary
Buprenorphine, classified as a semisynthetic opioid within the morphinan chemical category, serves as a versatile compound that acts as a modulator of opioid receptors with mixed partial agonist properties.
In higher doses, it is employed to address opioid addiction in individuals dependent on opioids. At lower doses, it finds application in managing moderate to acute pain in individuals who are not tolerant to opioids. At even lower doses, it aids in controlling moderate chronic pain.
Originally patented in 1965, Buprenorphine received approval for medical use in the United States in 1981. In 2017, there were 14.6 million prescriptions written for this medication in the United States. Furthermore, Buprenorphine is a widely utilized medication for treating opioid use disorders, including heroin addiction. It is worth noting that some individuals may misuse Buprenorphine for recreational purposes through methods such as injection or intranasal administration, seeking the euphoric effects it can produce.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 52485-79-7 |
|---|---|
| PubChem CID | 644073 |
| IUPHAR/BPS | 1670 |
| DrugBank | DB00921 |
| ChemSpider | 559124 |
| UNII | 40D3SCR4GZ |
| KEGG | D07132 |
| ChEBI | CHEBI:3216 |
| ChEMBL | ChEMBL511142 |
| CompTox Dashboard (EPA) | DTXSID2022705 |
| ECHA InfoCard | 100.052.664 |
| Chemical and physical data | |
| Formula | C29H41NO4 |
| Molar mass | 467.650 g·mol−1 |
Chemistry
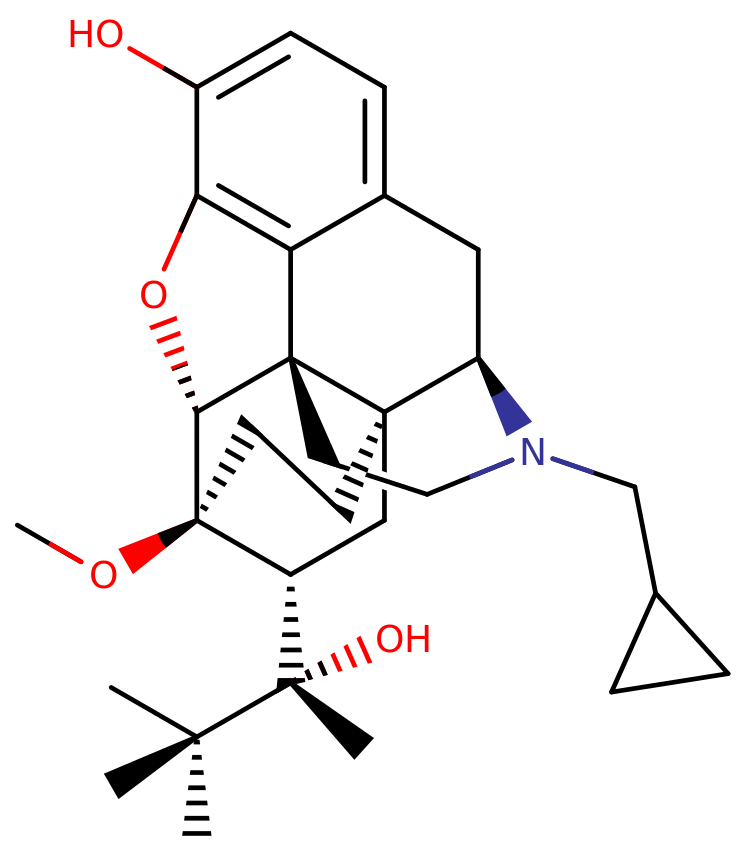
Buprenorphine represents a semi-synthetic compound derived from the opioid alkaloid thebaine, and like several other opioids, such as codeine or hydrocodone, it possesses a morphinan structure.
Within the class of buprenorphine and similar molecules, there exists a polycyclic core featuring three benzene rings fused in a zig-zag fashion, a configuration termed phenanthrene. Additionally, a fourth nitrogen-containing ring is fused to the phenanthrene at positions R9 and R13. Notably, buprenorphine, along with other morphinans, is characterized by an ether bridge connecting two of its rings, creating a linkage between the benzene and an opposing cyclohexane ring via an oxygen group.
What sets buprenorphine apart in the realm of human medical opioids is its inclusion of an additional fused ring connecting to the lower cyclohexane ring at positions R6 and R14. This distinctive structure is known as an endoethenotetrahydrooripavine backbone, a feature often found in veterinary opioids. Furthermore, buprenorphine exhibits a hydroxy group (OH-) substitution on the benzene ring and a methoxy group affixed to the lower cyclohexane ring. The backbone of buprenorphine also carries a methyl cyclopropyl moiety on its amino group. In proximity to its methoxy attachment, the cyclohexane ring is linked to R2 of a 2-butanol chain.
Pharmacology
Buprenorphine functions as a partial agonist of the μ-opioid receptor, displaying a binding affinity with a Ki of approximately 1.5 nM, in contrast to full agonists like morphine. Additionally, it serves as an antagonist of the κ-opioid receptor, boasting a binding affinity of Ki around 2.5 nM, and the δ-opioid receptor, with a binding affinity of Ki roughly at 6.1 nM. The significance of the ratio of these binding affinities becomes evident when comparing morphine’s binding ratio of 1:50:176 to Buprenorphine’s ratio of 15:25:61. This disparity implies that Buprenorphine is associated with a heightened side-effect profile. At the same time, its euphoric effects are notably low or even negligible. This is because, to achieve sufficient μ-opioid stimulation for euphoric effects, Buprenorphine must also bind to delta and kappa opioid receptors to a comparatively high extent.
Buprenorphine exerts its effects by engaging and activating the μ-opioid receptor, a mechanism stemming from the structural resemblance between opioids and naturally occurring endorphins within the body. These endorphins also interact with the μ-opioid receptor set. The structural mimicry of opioids to these natural endorphins underpins their capacity to induce euphoria, provide pain relief, and offer anxiolytic effects. This is due to the fact that endorphins play a role in diminishing pain, inducing drowsiness, and generating feelings of pleasure. Their release can be triggered by various stimuli such as pain, intense physical activity, orgasm, or general excitement.
Subjective effects
Disclaimer: The effects detailed below are derived from the Subjective Effect Index (SEI), a research compilation based on anecdotal user accounts and the personal assessments of PsychonautWiki contributors. Consequently, it is advisable to approach these effects with a healthy degree of skepticism.
Additionally, it is essential to recognize that these effects may not consistently manifest predictably or dependably. However, higher doses are more likely to elicit the complete range of effects. Furthermore, it should be noted that higher doses increase the likelihood of adverse effects, which may encompass addiction, severe harm, or even fatality ☠.
Physical:
- Pain Relief
- Physical Euphoria – The physical euphoria produced by this substance is comparatively less intense when juxtaposed with that of morphine or diacetylmorphine (heroin). This distinction arises from its status as a partial agonist of the μ-opioid receptor. The sensation itself is characterized by profound feelings of physical comfort, warmth, and bliss, which permeate the entire body.
- Itchiness
- Respiratory Depression – Buprenorphine induces milder respiratory depression in comparison to other opioids, owing to its status as a partial agonist of the μ-opioid receptor.
- Dizziness
- Sedation
- Decreased Heart Rate
- Constipation
- Cough Suppression
- Decreased Libido
- Difficulty Urinating
- Pupil Constriction
- Nausea – Buprenorphine tends to induce notably more nausea and vomiting than other opioids.
- Appetite Suppression
- Orgasm Suppression
Cognitive:
- Buprenorphine is often described as generating a general mental state characterized by euphoria, relaxation, anxiety alleviation, and pain relief.
- Cognitive Euphoria – The cognitive euphoria induced by this substance is comparatively less intense than that of morphine or diacetylmorphine (heroin), owing to its status as a partial agonist of the μ-opioid receptor. Nevertheless, it remains capable of producing intense and overwhelming bliss at higher doses with low tolerance. A potent and overpowering feeling of emotional bliss, contentment, and happiness typifies this sensation.
- Anxiety Suppression
Toxicity
Buprenorphine exhibits a relatively low toxicity in proportion to its dosage, typically with a ceiling dose ranging from 16mg to 32mg. Beyond this threshold, there is no significant escalation in respiratory depression, which is the primary cause of death in opioid overdoses, as it leads to respiratory failure. Consequently, increasing the buprenorphine dosage beyond this range does not amplify the risk of fatality in a manner similar to other μ-opioid receptor agonists. It is essential to bear in mind that irrespective of the ceiling dose, a critical distinction must be drawn between the dosage administered to opioid-naive individuals and those with prior opioid experience. Even modest doses in individuals lacking opioid tolerance can lead to unpleasant side effects like dizziness, loss of balance, and vomiting. Due to the prolonged half-life of buprenorphine, these side effects can persist for an extended duration in opioid-naive individuals, posing a risk of severe dehydration from uncontrollable vomiting.
Buprenorphine is frequently marketed under the brand name Suboxone, which also contains naloxone. Naloxone is not orally active except at higher doses, so when significant amounts of Suboxone are ingested, naloxone becomes effective and counteracts the effects of buprenorphine. This inclusion is designed to deter the misuse of Suboxone.
It is strongly advised to practice harm reduction principles when using this substance.
Tolerance and Potential for Addiction:
As with other opioids, prolonged use of buprenorphine can be moderately addictive and carries a high potential for abuse, leading to psychological dependence in some users. When addiction takes hold, cravings and withdrawal symptoms can arise if the substance is abruptly discontinued. Tolerance to various effects of buprenorphine develops with repeated and extended usage. The rate at which tolerance develops varies for different effects, with tolerance to constipation-inducing effects evolving particularly slowly. Consequently, users may need to administer progressively larger doses to achieve the same effects. Subsequently, it takes approximately 3 to 7 days for the tolerance to decrease by half and 1 to 2 weeks to return to baseline in the absence of further consumption. Buprenorphine also engenders cross-tolerance with all other opioids, implying that after using buprenorphine, other opioids will have a reduced impact.
Precipitated Withdrawal Syndrome:
Buprenorphine has the potential to induce withdrawal symptoms in individuals dependent on opiates. This phenomenon occurs because buprenorphine is only a partial agonist, lacking the substantial efficacy of a full agonist in activating the receptor. Additionally, it possesses a very high binding affinity for the μ-opioid receptor (Ki = 1.5nM), displacing other agonists that may still be attached when buprenorphine is consumed. It is essential to note that a common misconception suggests that naloxone, present in some buprenorphine formulations, is responsible for precipitated withdrawal syndrome. This is inaccurate, as naloxone has a lower binding affinity than buprenorphine and is inactive through most routes of administration.
Dangerous Interactions:
Warning: Many psychoactive substances, while reasonably safe when used in isolation, can become dangerous and potentially life-threatening when combined with certain other substances. The subsequent list highlights some known dangerous interactions (though it may not encompass all of them). Always engage in independent research (e.g., via Google, DuckDuckGo, PubMed) to confirm the safety of combining two or more substances. Some of these interactions have been sourced from TripSit.
- Alcohol: Both substances enhance each other’s ataxia and sedation, potentially leading to unexpected loss of consciousness at high doses. To prevent vomit aspiration from excess, individuals affected should be placed in the recovery position. Memory blackouts are likely.
- Amphetamines: Stimulants increase respiration rate, allowing for higher opiate doses than usual. If the stimulant wears off first, the opiate may overwhelm the user and cause respiratory arrest.
- Benzodiazepines: The central nervous system and respiratory depressant effects may be additively or synergistically present. The two substances strongly and unpredictably potentiate each other, often leading to unconsciousness. If unconscious, vomit aspiration is a risk if the person is not placed in the recovery position. Blackouts and memory loss are common.
- Cocaine: Stimulants increase respiration rate, enabling higher opiate doses than would typically be used. If the stimulant wears off first, the opiate may overcome the user and lead to respiratory arrest.
- DXM: Generally regarded as toxic. Observations include central nervous system depression, difficulty breathing, heart problems, and liver toxicity. Additionally, if one takes DXM, their opiate tolerance decreases slightly, potentially causing additional synergistic effects.
- GHB/GBL: These substances strongly and unpredictably potentiate each other, quickly leading to unconsciousness. While unconscious, there is a risk of vomit aspiration if the person is not placed in the recovery position.
- Ketamine: Both substances carry a risk of vomiting and unconsciousness. Suppose the user loses consciousness while under the influence; there is a severe risk of vomit aspiration if they are not placed in the recovery position.
- MAOIs: Combining monoamine oxidase inhibitors (MAOIs) with specific opioids has been linked to rare instances of severe adverse reactions. These reactions can be either excitatory or depressive. Excitatory reactions may include agitation, headaches, sweating, hyperpyrexia, flushing, shivering, myoclonus, rigidity, tremors, diarrhea, hypertension, tachycardia, seizures, and coma. In some cases, these reactions have resulted in death.
- MXE: MXE can enhance opioid effects but also increases the risk of respiratory depression and organ toxicity.
- Nitrous: Both substances amplify each other’s ataxia and sedation, which can lead to unexpected loss of consciousness at high doses. While unconscious, there is a risk of vomit aspiration if the individual is not placed in the recovery position. Memory blackouts are common.
- PCP: PCP may reduce opioid tolerance, increasing the risk of overdose.
- Tramadol: Increased risk of seizures. Tramadol is known to induce seizures, and it may have additive effects on seizure threshold when combined with other opioids. Central nervous system and respiratory depressant effects may also be additively or synergistically present.
- Grapefruit: While grapefruit is not psychoactive, it can influence the metabolism of specific opioids. Tramadol, oxycodone, and fentanyl primarily undergo metabolism by the enzyme CYP3A4, which is significantly inhibited by grapefruit juice. This may cause the drug to remain in the body for an extended period, potentially increasing toxicity with repeated doses. Methadone may also be affected. CYP2D6 metabolizes codeine and hydrocodone. Individuals taking medications that inhibit CYP2D6, or those with a genetic mutation that lacks the enzyme, will not respond to codeine, as it cannot be metabolized into its active product: morphine.
Legal status
In the European Union, buprenorphine can be prescribed either as a standalone medication or in combination with other substances, and it is approved for the treatment of opioid addiction.
Here is the legal status of buprenorphine in various countries:
- Austria: Buprenorphine is legal for medical use under the AMG (Arzneimittelgesetz Österreich) but is illegal when sold or possessed without a prescription under the SMG (Suchtmittelgesetz Österreich).
- Canada: Buprenorphine is categorized as a Schedule I substance in Canada and is available only with a valid prescription.
- Germany: Buprenorphine is regulated under Anlage III BtMG (Narcotics Act, Schedule III) since September 1, 1984. It can only be prescribed using a narcotic prescription form.
- Netherlands: Buprenorphine is classified as a List II drug under the Opium Law, with special rules governing its prescription and dispensation.
- Russia: Buprenorphine is considered a Schedule II controlled substance.
- Sweden: Buprenorphine is classified as a class IV controlled substance.
- Switzerland: Buprenorphine is a controlled substance specifically listed under Verzeichnis A, and its medicinal use is permitted.
- United States: Buprenorphine, whether used alone or in combination with naloxone (e.g., Suboxone), is classified as a Schedule III drug.
Before the approval of Suboxone in the United States for the treatment of opioid addiction, the Drug Addiction Treatment Act of 2000 was enacted. This legislation empowered the Secretary of Health and Human Services to grant a waiver to qualified physicians, allowing them to prescribe and administer narcotics from Schedules III-V for the treatment of drug addiction. Previously, this authority was limited to physicians working in specialized outpatient clinics for addiction treatment. The waiver necessitated physicians to complete an 8-hour training course and initially allowed them to treat only 10 patients using this approach. As of 2016, this limit has been raised to 275 patients.
FAQ
1. What is Buprenorphine? Buprenorphine is a medication used in the treatment of opioid addiction and as a pain reliever. It belongs to the opioid class of drugs but is unique in its action, acting as a partial agonist at the μ-opioid receptor.
2. How does Buprenorphine work? Buprenorphine alleviates withdrawal symptoms and cravings in individuals addicted to opioids. It attaches to the μ-opioid receptors in the brain, partially activating them, which reduces cravings without producing the intense euphoria of full opioid agonists like heroin or oxycodone.
3. Is Buprenorphine safe to use? When used as prescribed and under medical supervision, Buprenorphine is generally considered safe and effective. However, like any medication, it has potential side effects and should only be used as directed by a healthcare professional.
4. What are the common side effects of Buprenorphine? Common side effects may include constipation, nausea, headache, dizziness, and drowsiness. These effects usually diminish as the body adjusts to the medication.
5. Is Buprenorphine addictive? Buprenorphine itself has a lower potential for addiction compared to full opioid agonists, but it can be habit-forming when not used as prescribed. It is often used as a replacement therapy to manage opioid addiction and reduce the risk of illicit drug use.
6. How is Buprenorphine administered? Buprenorphine is available in various formulations, including sublingual tablets, sublingual films, and injections. The sublingual (under the tongue) form is the most common for addiction treatment.
7. Can I drive or operate heavy machinery while taking Buprenorphine? Buprenorphine can cause drowsiness and impair cognitive function in some individuals, especially when combined with other substances like alcohol. It is essential to be cautious when driving or operating machinery until you know how Buprenorphine affects you.
8. Can I stop taking Buprenorphine suddenly? Abruptly discontinuing Buprenorphine can lead to withdrawal symptoms. It is crucial to follow your healthcare provider’s guidance when tapering off the medication.
9. Can Buprenorphine be used during pregnancy? Buprenorphine is considered safer than full agonist opioids during pregnancy, but a healthcare professional should closely monitor its use during pregnancy. It may be used as part of a comprehensive treatment plan for pregnant individuals with opioid addiction.
10. Is Buprenorphine available over-the-counter (OTC)? No, Buprenorphine is a prescription medication, and you cannot purchase it over the counter. It should only be obtained and used under the supervision of a licensed healthcare provider.
11. Can Buprenorphine be abused or misused? While Buprenorphine has a lower abuse potential compared to other opioids, it can be misused. Some individuals may misuse it by taking higher doses than prescribed or using it to self-medicate without medical supervision.
12. Where can I find treatment with Buprenorphine for opioid addiction? You can find addiction treatment providers who prescribe Buprenorphine by contacting addiction treatment centers clinics, or seeking assistance from your healthcare provider. Telemedicine options may also be available.
13. Is it safe to drink alcohol while taking Buprenorphine? It is generally recommended to avoid alcohol while taking Buprenorphine, as it can increase the risk of side effects and impair your judgment and coordination.
14. Can Buprenorphine help with pain management? Yes, Buprenorphine can be prescribed for pain management, especially in situations where other opioids may not be suitable. It is often used for chronic pain or as an alternative to traditional opioids.
15. Can I switch from methadone to Buprenorphine? Transitioning from methadone to Buprenorphine can be done, but a healthcare professional should carefully manage it. A period of stabilization on a lower methadone dose may be required before starting Buprenorphine.
16. Is Buprenorphine covered by insurance? Most health insurance plans, including Medicaid and Medicare, typically cover buprenorphine. However, coverage may vary, so it’s advisable to check with your insurance provider for specific details.
17. How long should I take Buprenorphine for opioid addiction treatment? The duration of buprenorphine treatment varies from person to person. It is often recommended as a long-term maintenance therapy, but your healthcare provider will determine the appropriate duration based on your individual needs and progress in recovery.
References
- Combining Depressants: Risks to Be Aware Of Combining depressant substances, such as opioids, alcohol, and sedatives, can have severe and potentially life-threatening consequences. These substances slow down the central nervous system, leading to a range of adverse effects, including respiratory depression, impaired coordination, and cognitive deficits. It’s essential to be aware of these risks and exercise caution when using depressants.
- Bioavailability of Sublingual Buprenorphine In January 1997, a study by Mendelson et al. explored the bioavailability of sublingual buprenorphine. Sublingual administration of buprenorphine is a common route in addiction treatment. Understanding its bioavailability helps optimize dosing and treatment outcomes.
- Systemic Availability of Nasal Spray Buprenorphine Research by Eriksen et al. in November 1989 investigated the systemic availability of buprenorphine administered via nasal spray. This study sheds light on an alternative method of delivering buprenorphine and its effectiveness.
- Analogue-Based Drug Discovery Fischer and Ganellin’s work in 2006 on analogue-based drug discovery provides insights into the development of pharmaceuticals, including buprenorphine. Understanding the principles of drug discovery can help improve the effectiveness and safety of medications.
- Substance Abuse and Mental Health Services Administration (SAMHSA) SAMHSA plays a crucial role in substance abuse treatment and prevention. Accessing information from SAMHSA can provide valuable resources and guidance for individuals and healthcare professionals dealing with opioid addiction.
- Opioid Toxidrome and Grapefruit Juice Interaction A study published in March 2020, authored by Ershad et al., highlights the potential opioid toxidrome resulting from grapefruit juice consumption in individuals on methadone maintenance. Understanding drug interactions like these is essential for patient safety.
- EMA (European Medicines Agency) The European Medicines Agency, or EMA, is a regulatory authority responsible for evaluating and approving medications, including buprenorphine. Staying informed about EMA’s assessments can help ensure the safe use of medications in the European Union.
- Controlled Drugs and Substances Act in Canada The Controlled Drugs and Substances Act in Canada governs the legal framework for controlled substances, including buprenorphine. Comprehending the legal context is vital for those involved in the healthcare and regulatory aspects of these substances.
- German Narcotics Act (Anlage III BtMG) Germany’s Narcotics Act, specifically Anlage III BtMG, classifies and regulates substances like buprenorphine. Understanding these legal provisions is crucial for healthcare professionals and policymakers in Germany.
- Russian Government Resolution on Controlled Substances The Russian government’s resolution dated October 1, 2012, addresses the control of substances, including buprenorphine. Knowledge of such regulations is essential for compliance and safety in Russia.
- Swedish Medicines Agency (Läkemedelsverket) Regulations The Swedish Medicines Agency issues regulations concerning narcotic substances. These regulations, such as LVFS 1997:12, guide the appropriate use and handling of controlled substances in Sweden.
- Swiss Federal Chancellery’s Regulations on Controlled Substances Switzerland’s Federal Chancellery provides regulations on controlled substances, including buprenorphine. Comprehending these regulations is essential for healthcare and regulatory compliance in Switzerland.
- Impact of Policy Changes on the Opioid Epidemic Policy changes can have a significant impact on addressing the opioid epidemic. Understanding the evolving landscape of opioid-related policies, such as those made during the Obama administration, is crucial for shaping effective responses to this public health crisis.