Contents
Summary
Codeine, also known as 3-methylmorphine, is a naturally occurring opioid substance belonging to the morphinan class. It is found in extracts of the poppy plant, especially in Papaver bracteatum. Substances within this class are known for their effects, which include sedation, cough suppression, and the induction of euphoria when administered.
In opium, codeine ranks as the second most abundant alkaloid, constituting up to three percent of its composition.[citation needed] While codeine can be derived from natural sources, a semi-synthetic process is the primary means of producing pharmaceutical-grade codeine. It serves as the archetype for weak to midrange opioids alongside tramadol, dextropropoxyphene, dihydrocodeine, and hydrocodone.[citation needed]
Currently, codeine stands as the most widely used opiate globally, and it is one of the most frequently utilized drugs worldwide, as reported by various organizations, including the World Health Organization and its predecessor, the League of Nations. It is known for its effectiveness as an orally administered opioid analgesic and is recognized for its wide safety margin.
In recreational contexts, codeine is available over the counter, often as part of painkillers that mix it with other, potentially more toxic substances. Some individuals employ a cold water extraction technique to separate codeine from these mixtures.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 76-57-3 |
|---|---|
| PubChem CID | 5284371 |
| IUPHAR/BPS | 1673 |
| DrugBank | DB00318 |
| ChemSpider | 4447447 |
| UNII | UX6OWY2V7J |
| KEGG | C06174 |
| ChEBI | CHEBI:16714 |
| ChEMBL | ChEMBL485 |
| CompTox Dashboard (EPA) | DTXSID2020341 |
| ECHA InfoCard | 100.000.882 |
| Chemical and physical data | |
| Formula | C18H21NO3 |
| Molar mass | 299.370 g·mol−1 |
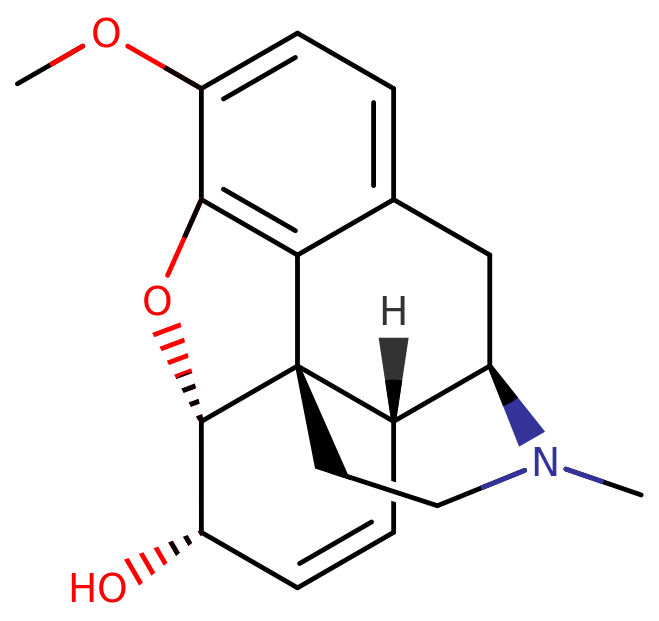
Chemistry
Codeine, also known as 3-methylmorphine, falls within the opioid class known as morphinans. Within this class, codeine and similar compounds share a common polycyclic structure consisting of three benzene rings fused in a zig-zag pattern referred to as phenanthrene. An additional nitrogen-containing ring is fused to the phenanthrene at positions R9 and R13, with the nitrogen atom located at R17 within the combined structure. This fundamental structure is termed morphinan.
Codeine, along with other morphinans, possesses an ether bridge connecting two of its rings, specifically linking R4 and R5 through an oxygen atom. It also features a hydroxy group (OH-) attached to R6 and a methyl group located on the nitrogen atom at R17.
Notably, on the same ring where the hydroxy group is situated, codeine exhibits a double bond that distinguishes it from dihydrocodeine. While codeine shares similarities with morphine, including the presence of an oxygen group at R3, in codeine, this oxygen group is substituted by a methyl group, forming a methoxy group.
In the realm of morphinans, codeine is closely related to other compounds like dihydrocodeine, heroin, ethylmorphine, hydrocodone, and oxycodone, sharing core structural features that define this class of opioids.
Pharmacology
Codeine, in its natural form, is not centrally active and requires initial conversion into morphine through first-pass metabolism, a process catalyzed by the cytochrome P450 enzyme CYP2D6. This transformation makes codeine a prodrug for morphine. Additionally, codeine can be metabolized into the inactive compound norcodeine via the CYP3A4 enzyme system. Both resulting forms are further modified into their respective 3-glucuronide conjugates.
A significant portion of the population exhibits variations in CYP2D6 enzyme production, leading to varying responses to codeine. Some individuals produce fewer CYP2D6 enzymes, resulting in diminished codeine effects compared to the average person. Conversely, higher CYP2D6 enzyme levels can lead to hypersensitivity to the drug. Certain methods to potentiate opioids, like consuming grapefruit juice before use, inhibit the CYP3A4 enzyme. This inhibition reduces the conversion of codeine into norcodeine, leaving more available for conversion into morphine.[citation needed]. Moreover, the combination of codeine with antihistamines like diphenhydramine can further hinder its metabolism into morphine.
There exists an upper limit, often referred to as the “ceiling dose,” for the amount of codeine that can be enzymatically converted into morphine during a single session. This ceiling dose is typically believed to be around 400mg. Consuming doses beyond this limit may lead to heightened side effects like itchiness and nausea, but it will not increase the feeling of euphoria.
The active metabolites of codeine, particularly morphine, exert their effects by binding to and activating opioid receptors, primarily the μ-opioid receptor. This mechanism occurs because opioids structurally resemble the body’s endogenous endorphins, which also interact with the μ-opioid receptor. The structural similarity to endorphins underlies the euphoria, pain relief, muscle relaxation, and anxiolytic effects of opioids. Endorphins are responsible for reducing pain, inducing sleepiness, and creating feelings of pleasure, often released in response to pain, strenuous exercise, orgasm, or general excitement.
Codeine itself is a weak ligand for opioid receptors, but its primary active metabolite, morphine, exhibits much stronger agonistic effects. Binding affinities (Ki)
- Mu opioid agonist – 589 nM
- Kappa opioid agonist – 18061 nM
- Delta opioid agonist – 11442 nM
Subjective effects
Disclaimer: The effects listed below are based on the Subjective Effect Index (SEI), which relies on open research literature derived from anecdotal user reports and personal analyses by contributors to PsychonautWiki. It is essential to approach these effects with a critical perspective.
It is also important to note that these effects may not occur predictably or consistently. Higher doses are more likely to encompass the full spectrum of effects. Furthermore, adverse effects become increasingly probable with higher doses, potentially including addiction, severe harm, or even fatality ☠.
Physical:
- General Head Space: Many describe the overall head space induced by codeine as characterized by euphoria, relaxation, anxiety relief, and pain alleviation.
- Pain Relief
- Physical Euphoria: The physical euphoria produced by this substance is considered less intense compared to that of morphine or diacetylmorphine (heroin) due to limitations in metabolic conversion. It involves an overwhelming sense of physical comfort, warmth, and bliss that spreads throughout the body.
- Itchiness: Codeine tends to induce greater itchiness due to increased histamine release compared to other opioids.
- Respiratory Depression: At low to moderate doses, codeine may mildly to moderately slow down breathing without noticeable impairment. At high doses or overdoses, it can lead to shortness of breath, abnormal breathing patterns, semi-consciousness, or unconsciousness. Severe overdoses may result in coma or death without immediate medical intervention.
- Sedation: Higher doses of codeine can induce sedation, and it is notably more sedating than oxycodone and hydrocodone.
- Appetite Suppression
- Constipation
- Cough Suppression
- Decreased Libido
- Difficulty Urinating
- Nausea: This effect is more likely to occur at high doses or when users do not pace themselves adequately.
- Orgasm Suppression
- Pupil Constriction
Cognitive:
- Cognitive Euphoria: Codeine’s cognitive euphoria is considered less intense compared to that of morphine or diacetylmorphine (heroin) due to metabolic limitations. However, at higher doses with low tolerance, it can produce powerful feelings of emotional bliss, contentment, and happiness.
- Anxiety Suppression
- Thought Deceleration
- Compulsive Redosing: It is essential to note that redosing codeine will not intensify its effects.
- Dream Potentiation
- Sleepiness
Visual:
Suppressions
- Double Vision: At high doses, codeine may cause uncontrolled eye defocusing and refocusing, resulting in a blurred effect and double vision that persists regardless of eye focus. This can be intense to the point of making reading or driving impossible.
Hallucinatory States
- Internal Hallucination: Heavy dosage nodding may lead to a state of semi-consciousness and hypnagogia, resulting in dream-like states and imagery up to level 3. Ill-defined geometric patterns often accompany these experiences.
1. Low Toxicity: Codeine has a relatively low toxicity relative to its dose. However, long-term use can lead to potential side effects, including diminished libido, apathy, and memory loss.
2. Allergic Reactions: Some individuals may experience allergic reactions to codeine, which can manifest as skin swelling and rashes.
3. Lethal Combinations: Codeine can be potentially lethal when combined with depressants such as alcohol or benzodiazepines. The interaction can lead to synergistic effects, increasing the risk of unconsciousness and memory blackouts.
Harm Reduction Practices: It is strongly recommended to practice harm reduction when using this drug to minimize risks associated with its use.
Tolerance and Addiction Potential
4. Moderately Addictive: Chronic use of codeine is moderately addictive, with a high potential for abuse. It can lead to psychological dependence in certain users.
5. Cravings and Withdrawal: Addiction to codeine may result in cravings and withdrawal symptoms if usage is abruptly discontinued.
6. Tolerance Development: Tolerance to many of codeine’s effects develops with prolonged and repeated use. The rate at which tolerance develops varies for different effects. For instance, tolerance to the constipation-inducing effects develops particularly slowly.
7. Cross-tolerance: Codeine can create cross-tolerance with all other opioids, meaning that after using codeine, the effects of other opioids will be reduced.
Dangerous Interactions
8. Caution: Many psychoactive substances that are safe when used alone can become dangerous or life-threatening when combined with other substances. It is crucial to conduct independent research to ensure the safety of combining two or more substances.
9. Known Dangerous Interactions: Some known dangerous interactions include:
Toxicity
- Alcohol: Combining alcohol and codeine can potentiate ataxia and sedation, leading to unexpected loss of consciousness, with memory blackouts being likely.
- Amphetamines: Stimulants can increase respiration rates, allowing for higher opioid doses. If the stimulant wears off first, it may result in respiratory arrest.
- Benzodiazepines: The combination of benzodiazepines and codeine can lead to strong and unpredictable central nervous system and respiratory-depressant effects, rapidly causing unconsciousness. Memory loss and blackouts are likely.
- Cocaine: Cocaine, a stimulant, can increase respiration rates, allowing for higher opioid doses. If the stimulant effect wanes first, respiratory arrest may occur.
- DXM: DXM is generally considered toxic when combined with opioids. It can lead to central nervous system depression, difficulty breathing, heart issues, and liver toxicity. Additionally, DXM may lower opioid tolerance, resulting in additional synergistic effects.
- GHB/GBL: Combining GHB or GBL with codeine can lead to strong and unpredictable potentiation, potentially resulting in unconsciousness. Vomit aspiration is a risk while unconscious.
- Ketamine: Both substances carry the risk of vomiting and unconsciousness. Falling unconscious while under their influence can pose a severe risk of vomit aspiration without proper positioning.
- MAOIs: Co-administering monoamine oxidase inhibitors (MAOIs) with opioids can result in rare but severe adverse reactions. These interactions can be excitatory or depressive and may include agitation, headache, hyperpyrexia, seizures, and even coma or death.
- MXE: MXE can potentiate opioid effects but also increase the risk of respiratory depression and organ toxicity.
- Nitrous: Combining nitrous oxide and codeine can potentiate ataxia and sedation, potentially leading to loss of consciousness. Unconsciousness while under the influence poses a risk of vomit aspiration.
- PCP: PCP may reduce opioid tolerance, increasing the risk of overdose.
- Tramadol: Combining tramadol with opioids may increase the risk of seizures, as tramadol itself is known to induce seizures. This combination may also potentiate central nervous system and respiratory-depressant effects.
- Grapefruit: While not psychoactive itself, grapefruit can affect the metabolism of certain opioids. This can lead to prolonged drug clearance, increased toxicity with repeated doses, and potentially reduced efficacy of the opioid. It particularly affects opioids metabolized by CYP3A4, such as tramadol, oxycodone, and fentanyl.
Genetic Variations: People taking codeine should be aware that its metabolism involves the CYP2D6 enzyme. Individuals with genetic variations or those taking medicines that inhibit CYP2D6 may not respond to codeine effectively, as it cannot be metabolized into its active product, morphine.
Legal status
Australia: In Australia, codeine preparations are classified as Schedule 4 medications (Prescription Only) when combined with other substances. Pure codeine preparations, such as codeine phosphate tablets or codeine phosphate linctus, require a prescription and are categorized as Schedule 8 drugs (Controlled Drug, Possession without authority illegal). Schedule 8 drugs have strict restrictions to prevent misuse and dependence. Unauthorized possession of Schedule 8 drugs is a criminal offense, with penalties varying by state.
Austria: In Austria, codeine is legal for medical use under the AMG (Arzneimittelgesetz Österreich) but illegal for sale or possession without a prescription under the SMG (Suchtmittelgesetz Österreich).
Canada: Codeine is available over the counter in Canada but with certain restrictions. It is typically sold in combination tablets, containing no more than 8 mg per dosage unit and combined with at least two other active ingredients. Sales are made behind the counter, usually to individuals aged 18 and over, following a brief consultation at the pharmacist’s discretion. Preparations with higher codeine content or fewer active ingredients require a prescription.
Denmark: In Denmark, codeine is available over the counter with a maximum of 9.6 mg per mixture. Stronger preparations necessitate a prescription for legal possession.
Finland: In Finland, codeine cough syrups with a maximum strength of 1 mg/ml are available over the counter. Codeine in pill form requires a prescription. Stronger cough syrups and combination painkillers with codeine (e.g., codeine with paracetamol or ibuprofen) also require prescriptions.
France: In France, most codeine-containing preparations do not require a doctor’s prescription. Examples include Néocodion (cough pills and syrup), Codoliprane (codeine with paracetamol), Prontalgine, and Migralgine (codeine, paracetamol, and caffeine). However, codeine was reclassified as a controlled substance (Schedule 2) in 2017, making it available by prescription only.
Germany: Codeine is a controlled substance listed in Anlage III of the BtMG. It can only be prescribed on a narcotic prescription form. Preparations containing up to 2.5% or 100 mg codeine per unit can be prescribed on a regular prescription unless intended for individuals with alcohol or drug dependencies.
Greece: Codeine is considered an illegal drug in Greece. Only individuals with a doctor’s prescription can legally possess it.
Hong Kong: In Hong Kong, codeine is regulated under the Dangerous Drugs Ordinance. Only health professionals and researchers at universities can use it legally. Pharmacists can provide codeine under prescription. However, codeine is available without a prescription from licensed pharmacists in doses up to 0.1%.
Iceland: In Iceland, preparations for paracetamol and codeine require a prescription.
India: Codeine preparations require a prescription in India. Paracetamol and codeine preparations are available, as well as codeine in some cough syrups.
Iran: In Iran, codeine preparations are typically combined with paracetamol and can be purchased over the counter. Despite recreational use, authorities allow codeine to be sold without a doctor’s prescription, usually verifying the buyer’s age.
Ireland: Codeine remains an over-the-counter drug in Ireland, with a limit of 12.8 mg per pill. Stronger codeine products are available by prescription only.
Italy: Codeine tablets or preparations require a prescription in Italy. Paracetamol and codeine combinations are available as Co-Efferalgan and Tachidol.
Japan: Codeine and similar agents in combination with non-opioid analgesics, antihistamines, and other substances can be purchased over the counter in Japan, up to a ceiling of 10 mg per dosage.
Maldives: In the Maldives, codeine is banned unless individuals have a notarized and authenticated doctor’s prescription.
Poland: Codeine is listed in “Wykaz środków odurzających i substancji psychotropowych” group ” II-N,” which means it’s legal for scientific and medical purposes. It is available over the counter in doses of 15 mg in combination with 500 mg paracetamol or 300 mg sulfogaiacol. Pharmacy workers can refuse sales if they suspect misuse.
Romania: In Romania, codeine is sold over the counter when combined with another active ingredient, up to 12.8 mg per unit. Amounts exceeding 12.8 mg require a prescription.
Russia: OTC availability of codeine products in Russia was revoked nationwide in 2012 due to concerns over illicit desomorphine synthesis (Krokodil method).
Spain: Codeine tablets or preparations require a prescription in Spain, although this regulation may not always be enforced.
Sri Lanka: Codeine preparations are available as over-the-counter pharmacy medicines in Sri Lanka. The most common preparation is Panadeine, containing 500 mg of paracetamol and 8 mg of codeine.
Sweden: Codeine is classified as Schedule III under Swedish law, available only by prescription.
Switzerland: Codeine is a controlled substance specifically named under Verzeichnis A in Switzerland. Medicinal use is permitted. Some preparations containing codeine are included in Verzechnis C, while certain ones are excluded.
Turkey: Codeine is a ‘red prescription’ only substance in Turkey, and it is illegal to sell or possess it without a prescription.
United Arabian Emirates: In the United Arab Emirates, codeine and other medicines are strictly regulated. They are banned without a notarized and authenticated doctor’s prescription. Violators may face deportation or imprisonment.
United Kingdom: In the United Kingdom, codeine is classified under the Misuse of Drugs Act 1971. It is a Class B controlled substance or a Class A drug when prepared for injection. Possession of controlled substances without a prescription is a criminal offense. However, certain codeine preparations are exempt under Schedule 5 of the Misuse of Drugs Regulations 2001, allowing possession without a prescription as long as they contain at least one other active or inactive ingredient and the dosage does not exceed 100 mg or 2.5% concentration in liquid preparations.
United States: In the United States, codeine is regulated by the Controlled Substances Act. Its classification varies based on the product. Pure codeine products for pain relief containing codeine alone or exceeding 90 mg per dosage unit are Schedule II controlled substances. Tablets combining codeine with aspirin or acetaminophen (paracetamol) for pain relief are listed as Schedule III. Cough syrups may be Schedule III or V, depending on the formula.
FAQ
- What is Codeine?
- Codeine is an opioid medication used to relieve mild to moderate pain and suppress coughs. It’s derived from the opium poppy and belongs to the class of drugs known as opioids or narcotics.
- How Does Codeine Work?
- Codeine works by binding to specific receptors in the brain and spinal cord, known as opioid receptors. This action alters the perception of pain and can also lead to feelings of relaxation and euphoria.
- Is Codeine Available Over the Counter?
- The availability of codeine varies by country. In some places, it can be obtained without a prescription in low-dose formulations, often combined with other pain relievers or cough suppressants. However, stronger formulations typically require a doctor’s prescription.
- What Are Common Uses for Codeine?
- Codeine is commonly used to relieve mild to moderate pain, such as that caused by injuries or dental procedures. It’s also found in some cough syrups as a cough suppressant.
- What Are the Side Effects of Codeine?
- Common side effects of codeine use may include drowsiness, dizziness, constipation, and nausea. Some people may also experience itching or sweating. Serious side effects, such as difficulty breathing or irregular heartbeat, are rare but require immediate medical attention.
- Is Codeine Addictive?
- Yes, codeine has the potential for addiction, especially when used for an extended period or in high doses. It’s important to use it only as prescribed by a healthcare professional.
- How Long Does Codeine Stay in Your System?
- The duration of codeine’s presence in your system can vary depending on factors like your metabolism and the formulation of the drug. Typically, it can be detected in urine for 1-2 days but may be detected longer in chronic users.
- Can You Drive or Operate Machinery While Taking Codeine?
- Codeine can cause drowsiness and impair your ability to drive or operate machinery. It’s important to avoid these activities until you know how codeine affects you individually.
- Is It Safe to Drink Alcohol While Taking Codeine?
- Combining alcohol with codeine can increase the risk of side effects, including extreme drowsiness and respiratory depression. It’s generally not recommended to consume alcohol while using codeine.
- What Should I Do If I Suspect an Overdose on Codeine?
- If you suspect a codeine overdose, which may include symptoms like slow or shallow breathing, extreme drowsiness, or loss of consciousness, seek emergency medical attention immediately.
- Can Pregnant or Nursing Women Use Codeine?
- Pregnant or nursing women should consult their healthcare provider before using codeine. It can pass into breast milk and potentially affect the baby. The use of codeine during pregnancy is generally discouraged.
- Is It Safe to Stop Taking Codeine Suddenly?
- Abruptly stopping codeine after prolonged use can lead to withdrawal symptoms. If you wish to discontinue codeine, it’s advisable to consult your healthcare provider to develop a safe tapering plan.
- Can Codeine Interact with Other Medications?
- Yes, codeine can interact with other medications, including other opioids, sedatives, and certain antidepressants. It’s crucial to inform your healthcare provider of all medications you are taking to avoid potentially dangerous interactions.
- Can I Get Codeine Online Without a Prescription?
- It is not safe to obtain codeine or any prescription medication online without a valid prescription from a licensed healthcare provider. Purchasing medications from unverified sources can be risky and illegal.
- Is Codeine Legal Worldwide?
- Codeine’s legal status varies from country to country. While it may be available over the counter in some places, in others, it requires a prescription or is heavily regulated.
Always consult with a healthcare professional or pharmacist for specific guidance regarding the use of codeine and to address any questions or concerns you may have.
References
- Hull, J. H., Findlay, J. W., Rogers, J. F., Welch, R. M., Butz, R. F., Bustrack, J. A. (November 1982). “An evaluation of the effects of smoking on codeine pharmacokinetics and bioavailability in normal human volunteers”. Drug Intelligence & Clinical Pharmacy. 16 (11): 849–854. doi:10.1177/106002808201601107. ISSN 0012-6578.
- Dicpinigaitis, P. V., Morice, A. H., Birring, S. S., McGarvey, L., Smith, J. A., Canning, B. J., Page, C. P. (April 2014). “Antitussive Drugs—Past, Present, and Future”. Pharmacological Reviews. 66 (2): 468–512. doi:10.1124/pr.111.005116. ISSN 0031-6997.
- Girennavar, B., Jayaprakasha, G. K., Patil, B. S. (October 2007). “Potent inhibition of human cytochrome P450 3A4, 2D6, and 2C9 isoenzymes by grapefruit juice and its furocoumarins”. Journal of Food Science. 72 (8): C417–421. doi:10.1111/j.1750-3841.2007.00483.x. ISSN 1750-3841.
- Lurcott, G. (1998). “The effects of the genetic absence and inhibition of CYP2D6 on the metabolism of codeine and its derivatives, hydrocodone and oxycodone”. Anesthesia Progress. 45 (4): 154–156. ISSN 0003-3006.
- Thompson, C. M., Wojno, H., Greiner, E., May, E. L., Rice, K. C., Selley, D. E. (1 February 2004). “Activation of G-Proteins by Morphine and Codeine Congeners: Insights to the Relevance of O- and N-Demethylated Metabolites at μ- and δ-Opioid Receptors”. Journal of Pharmacology and Experimental Therapeutics. 308 (2): 547–554. doi:10.1124/jpet.103.058602. ISSN 0022-3565.
- Ershad, M., Cruz, M. D., Mostafa, A., Mckeever, R., Vearrier, D., Greenberg, M. I. (March 2020). “Opioid Toxidrome Following Grapefruit Juice Consumption in the Setting of Methadone Maintenance”. Journal of Addiction Medicine. 14 (2): 172–174. doi:10.1097/ADM.0000000000000535. ISSN 1932-0620.