In the internet age, the accessibility of various substances, including designer drugs like 2C-B, has grown exponentially. This surge in availability has given rise to many online vendors claiming to offer 2C-B for sale. While some may appear legitimate, it is essential to critically evaluate these sellers regarding safety, legality, and ethical considerations.
Contents
- 0.1 Legality and Compliance:
- 0.2 Transparency and Accountability:
- 0.3 Safety and Health Risks:
- 0.4 Ethical Considerations:
- 1 Summary
- 2 History and culture
- 3 Chemistry
- 4 Pharmacology
- 5 Subjective effects
- 6 Reagent results
- 7 Toxicity
- 8 Legal status
- 9 FAQ
- 9.1 1. What is 2C-B?
- 9.2 2. How is 2C-B typically consumed?
- 9.3 3. What are the effects of 2C-B?
- 9.4 4. How long does a 2C-B trip last?
- 9.5 5. Is 2C-B legal?
- 9.6 6. Is 2C-B safe to use?
- 9.7 7. Can 2C-B be used for therapeutic purposes?
- 9.8 8. Are there any known interactions or risks with 2C-B?
- 9.9 9. Can 2C-B be addictive?
- 9.10 10. Is there a safe dosage for 2C-B?
- 9.11 11. Can 2C-B be tested for purity?
- 9.12 12. What should I do in case of a bad trip on 2C-B?
- 10 References
Legality and Compliance:
One of the primary concerns with online 2C-B vendors is the legal aspect. In many countries, 2C-B is considered a controlled substance or a scheduled drug, making its sale and possession illegal. Buyers should be cautious when dealing with sellers promoting 2C-B for sale, as engaging in such transactions can have severe legal consequences.
Transparency and Accountability:
Research chemical sellers often lack transparency and accountability in regulated markets. Many operate anonymously and may not provide sufficient information regarding their products’ quality, purity, or source. This lack of transparency poses severe risks to consumers, as they have no way of verifying the authenticity or safety of the substances they buy.
Safety and Health Risks:
Purchasing 2C-B from online vendors can expose buyers to significant health risks. The absence of quality control measures can lead to the distribution of impure or contaminated substances, potentially causing adverse effects, overdoses, or long-term health consequences.
Ethical Considerations:
Supporting online vendors selling 2C-B and other research chemicals raises ethical questions. These substances often bypass rigorous safety and efficacy testing conducted in pharmaceutical research. Buyers inadvertently contribute to a market that operates in a legal gray area and lacks ethical oversight by purchasing from such vendors.
Summary
4-Bromo-2,5-dimethoxyphenethylamine, commonly known as 2C-B or by various street names like Nexus, Bromo Mescaline, BDMPEA, and Venus, is a novel psychedelic compound belonging to the phenethylamine class. It is the most prominent member of the 2C-x family, sharing structural similarities with the classical psychedelic mescaline. While the precise mechanism of action remains incompletely understood, it is believed that 2C-B primarily exerts its effects by binding to serotonin receptors.
In 1974, American chemist Alexander Shulgin made the significant discovery of 2C-B while exploring psychedelic phenethylamines derived from mescaline. During the 1970s, a select group of American psychotherapists found therapeutic value in 2C-B due to its brief duration, limited side effects, and relatively mild nature. It gained a reputation as a valuable substance for therapeutic purposes.
However, its recreational use soon emerged, and briefly, it was commercially available under names such as “Erox” and “Nexus” in specialty shops and adult video stores. In 1995, it was classified as a controlled substance at the federal level.
Subjective effects of 2C-B encompass open and closed-eye visuals, time distortion, euphoria, and ego dissolution. Users have described it as a moderately warm, colorful, and highly sensual experience. Unlike tryptamines like LSD or psilocybin, which can induce profound introspection, 2C-B tends to emphasize the visual and tactile aspects of the psychedelic experience.
Lower doses (under 15 mg) are reported to enhance sensory perception and aesthetics, akin to MDMA, to some extent. In contrast, higher doses produce a distinct and profound psychedelic experience, affecting the user’s mental state.
While 2C-B appears well-tolerated physiologically, more research is needed to fully understand its safety profile. It is generally believed to share similarities with classical psychedelics, characterized by low abuse potential and toxicity. However, it is essential to acknowledge the potential for adverse psychological reactions, particularly in individuals predisposed to mental disorders. These reactions may include severe anxiety, paranoia, delusions, or psychosis. Therefore, responsible use and caution are paramount when engaging with 2C-B or any psychedelic substance.
| Identifiers | |
|---|---|
| show IUPAC name | |
| CAS Number | 66142-81-2 |
| PubChem CID | 98527 |
| DrugBank | DB01537 |
| ChemSpider | 88978 |
| UNII | V77772N32H |
| ChEBI | CHEBI:189669 |
| ChEMBL | ChEMBL292821 |
| CompTox Dashboard (EPA) | DTXSID10216332 |
| ECHA InfoCard | 100.164.088 |
| Chemical and physical data | |
| Formula | C10H14BrNO2 |
| Molar mass | 260.131 g·mol−1 |
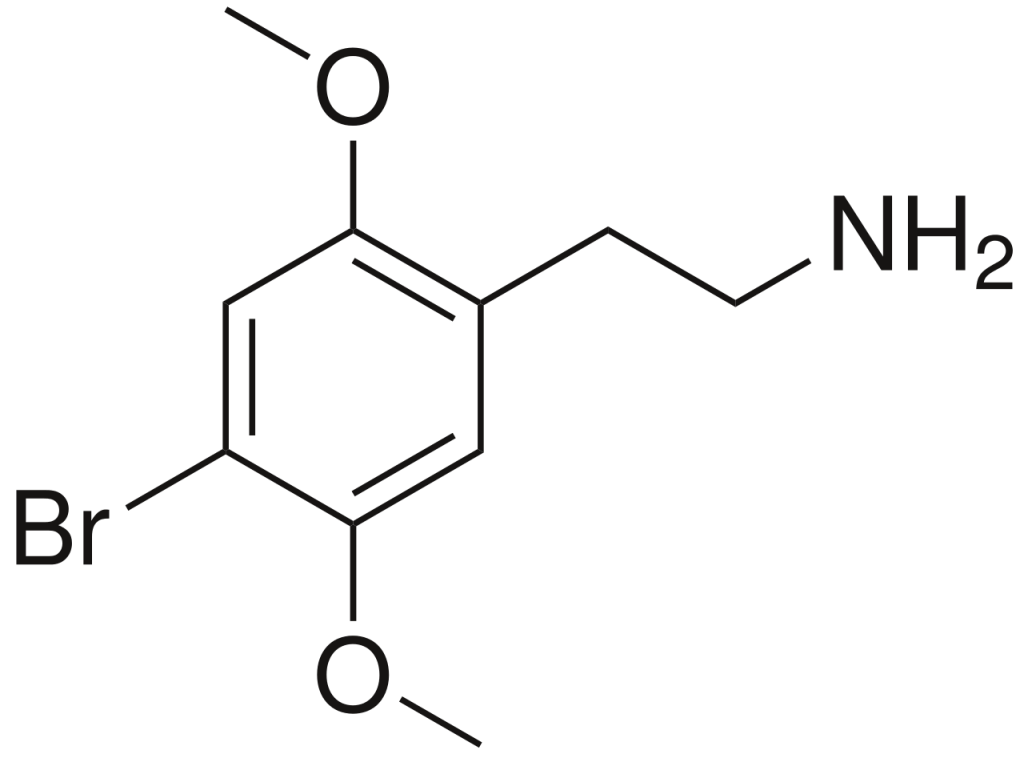
History and culture
1974 American chemist and pioneering psychedelic researcher Alexander Shulgin synthesized and investigated 2C-B for its psychoactive properties. His exploration focused on novel psychedelic compounds with chemical structures reminiscent of mescaline.
Shulgin documented his discoveries in the 1991 book “PiHKAL,” where he categorized 2C-B among the “magical half-dozen” of psychedelic phenethylamines, a selection he considered particularly significant. This esteemed list included mescaline, DOM, 2C-B, 2C-E, 2C-T-2, and 2C-T-7. Shulgin frequently expressed his personal preference for 2C-B as his favored psychedelic experience.
During the 1970s, a few psychotherapists in the United States began utilizing 2C-B in their patient treatments. They reported that the compound facilitated warm, empathetic connections between therapists and patients, assisting in dismantling ego defenses and inner resistances. This, in turn, allowed patients to access suppressed emotions and repressed memories. The gentle and manageable effects of 2C-B and its short duration and limited side effects made it an appealing choice for therapeutic contexts.
Subsequently, 2C-B transcended its therapeutic origins and found popularity within the recreational drug scene. It gained favor as a substitute for MDMA in rave and club settings due to its comparatively mild comedown and ability to induce a lucid, euphoric state of mind.
In the 1980s and early 1990s, several foreign companies legally manufactured 2C-B, marketing it under brand names like “Nexus,” “Erox,” and “Performax.” These products were promoted to address issues like impotence, frigidity, and decreased libido. They were available at adult book and video stores, “head” shops, and even certain nightclubs. Notably, the DEA documented the distribution of yellow pills in Miami, Florida, marketed as an aphrodisiac.
In the United States, 2C-B gained prominence as an alternative to MDMA after MDMA was classified as a Schedule I substance in 1985. Consequently, 2C-B was also placed in Schedule I in 1995. Its resurgence in the 2000s was facilitated by the emergence of the “research chemicals” and “designer drugs” scene, along with the availability of darknet markets.
Remarkably, 2C-B was legally obtainable in Southern Africa from 1993 to early 1996, where it was marketed as “Ubulawu Nomathotholo,” loosely translating to “Medicine of the Singing Ancestors.” It was positioned as a remedy for traditional healers known as Sangomas.
Chemistry
2C-B, scientifically known as 2,5-dimethoxy-4-bromophenethylamine, falls into the category of substituted phenethylamines. These compounds are a class of organic substances built upon the phenethylamine structure, characterized by a phenyl ring linked to an amino (NH2) group via an ethyl chain. In the case of 2C-B, it exhibits methoxy functional groups (CH3O-) attached to carbons R2 and R5 and a bromine atom affixed to carbon R4 within the phenyl ring.
Furthermore, 2C-B is a member of the 2C family of phenethylamines, all sharing the common feature of methoxy groups located at the 2 and 5 positions of the benzene ring.
Pharmacology
In contrast to most psychedelics, 2C-B has demonstrated a unique pharmacological profile as a serotonin 5-HT2A receptor with low efficacy, functioning as either a partial agonist or, in some cases, a full antagonist. This intriguing property implies that the effects elicited by 2C-B users are primarily mediated through the 5-HT2C receptor. Additionally, research indicates that 2C-B can elevate dopamine levels in the brains of rats, potentially contributing to its psychoactive properties.
Nevertheless, the precise role of these interactions and how they culminate in the psychedelic experience remains an enigma, presenting an ongoing challenge for scientific understanding.

Subjective effects
Disclaimer: The effects mentioned below are drawn from the Subjective Effect Index (SEI), a research database based on anecdotal user accounts and the evaluations of contributors to PsychonautWiki. Therefore, it is advisable to approach these effects with a degree of skepticism.
It’s crucial to understand that these effects may not consistently manifest and can vary widely from person to person. Higher doses are more likely to induce the full range of effects but also increase the risk of adverse consequences, including addiction, severe harm, or even fatality ☠.
Physical:
- Stimulation: 2C-B is often described as highly energetic and stimulating, akin to MDMA. This stimulation may become more pronounced at higher doses, encouraging physical activities like dancing.
- Spontaneous Bodily Sensations: The “body high” associated with 2C-B is often regarded as one of the most intricate psychedelics. It combines elements of MDMA, 2C-E, and LSD, typically beginning with a warm, gentle glow that can evolve into intense euphoria.
- Nausea: Mild to extreme nausea can occur at moderate to high doses. This discomfort may subside after vomiting or gradually diminish as the peak effects occur.
- Appetite Suppression: While not as potent as stimulants like MDMA, 2C-B often leads to reduced appetite.
- Bodily Control Enhancement
- Increased Blood Pressure (citation needed)
- Increased Bodily Temperature (citation needed)
- Increased Heart Rate (citation needed)
- Increased Perspiration
- Dehydration (citation needed)
- Pupil Dilation
- Tactile Enhancement
- Teeth Grinding (citation needed) – This effect is typically milder than that associated with MDMA.
Visual:
- Enhancements
- Color Enhancement
- Magnification
- Pattern Recognition Enhancement
- Visual Acuity Enhancement
- Distortions
- Drifting (Melting, Flowing, Breathing, and Morphing): This effect is characterized by detailed, slow, smooth motions, static appearances, and cartoon-like styles compared to other psychedelics.
- After Images
- Colour Shifting
- Depth Perception Distortions
- Recursion
- Scenery Slicing
- Symmetrical Texture Repetition
- Tracers
- Geometry: The visual geometry of 2C-B is likened more to LSD than to 2C-E, psilocin, or ayahuasca. It is described as unstructured, algorithmic, intricate, large, fast, smooth, colorful, glossy, sharp, and angular.
- Hallucinatory States: While 2C-B can produce a range of hallucinatory states, they tend to be infrequent and inconsistent at higher doses, more common at lower doses, and include transformations, machine scapes, and internal hallucinations.
Cognitive:
- Insightful and Normal Thought Processes: Even at moderate to high doses, the cognitive effects of 2C-B are often described as both insightful and relatively typical in thought processes, falling somewhere between LSD and MDMA.
- Empathy, Affection, and Sociability Enhancement: While slightly milder than MDMA, 2C-B promotes sociability, love, and empathy, offering lasting therapeutic effects.
- Analysis Enhancement: This introspective effect primarily manifests in non-social settings when the user is alone.
- Anxiety and Paranoia: Though possible at most doses, these effects are generally less intense than those associated with classic psychedelics like LSD or psilocybin.
- Conceptual Thinking
- Delusion
- Creativity Enhancement
- Emotion Enhancement
- Immersion Enhancement
- Novelty Enhancement
- Increased Libido: Coupled with heightened bodily sensations and stimulation, 2C-B can make sexual activity pleasurable.
- Increased Music Appreciation
- Increased Sense of Humor
- Laughter Fits: 2C-B has been reported to induce sudden, intense fits of laughter.
- Memory Suppression
- Ego Death
- Personal Bias Suppression
- Rejuvenation
- Thought Acceleration
- Thought Connectivity
- Time Distortion
- Wakefulness
Auditory:
- Auditory Enhancement
- Auditory Distortion
- Auditory Hallucination
Multi-Sensory:
- Synaesthesia: Although rare and non-reproducible in its whole form, this effect may become more likely at higher doses.
Transpersonal:
- While 2C-B has the potential to induce transpersonal states, they are generally reported as less consistent or intense compared to classic psychedelics such as psilocybin mushrooms, mescaline, LSD, or DMT. These effects include existential self-realization, spirituality enhancement, unity, and interconnectedness.
Combination:
- Cannabis: Combining cannabis with 2C-B can significantly intensify and prolong sensory and cognitive effects. However, this combination also increases the risk of anxiety, confusion, and psychosis.
- Dissociatives: When used with dissociatives, 2C-B often amplifies geometry, euphoria, dissociation, and hallucinatory effects.
- Nitrous: Nitrous oxide, commonly combined with psychedelics, synergizes with 2C-B, potentially inducing rapid “ego death” and euphoria.
- MDMA: MDMA strongly potentiates the physical, visual, and cognitive effects of 2C-B, offering unique body highs, altered states of consciousness, and colorful visuals. Caution is advised when combining these substances due to their unpredictable synergy, and lower doses are recommended.
- Alcohol: Alcohol can intensify the disinhibiting and euphoric effects of 2C-B, but it may also lead to dehydration, nausea, and physical fatigue. Heavy drinking is discouraged.
- Benzodiazepines: Depending on the dosage, benzodiazepines can reduce the intensity of cognitive, physical, and visual effects, potentially halting “bad trips.” However, this may come at the cost of memory loss and decreased trip intensity.
- Psychedelics: Combining 2C-B with other psychedelics can synergize their physical, cognitive, and visual effects, though the outcome is unpredictable. Caution is advised, and lower doses are recommended for this combination.
Please exercise extreme caution and consider potential risks when combining substances.
Reagent results
Exposing compounds to the reagents gives a colour change which is indicative of the compound under test.
| Marquis | Mecke | Mandelin | Liebermann | Froehde | Robadope |
|---|---|---|---|---|---|
| Yellow – Green | Yellow – Olive brownish | green | Yellow – Black | Yellow – Green | Slow pink |
| Ehrlich | Hofmann | Simon’s | Scott | Folin | |
| No reaction | No reaction | No reaction | No reaction | (Light) purple | |
Toxicity
The toxicity and long-term health implications of recreational 2C-B use remain unexplored in scientific research, and the precise toxic dosage remains undisclosed.
Based on anecdotal accounts, no discernible adverse health consequences appear to be associated with infrequent or low to moderate doses of 2C-B when used in isolation. However, it’s essential to acknowledge that nothing can be guaranteed absolutely.
It is strongly recommended that individuals employ harm-reduction strategies when engaging with this substance.
Neurotoxicity:
2C-B at typical doses is unlikely to induce neurotoxic effects. By rough estimations derived from data involving rat cortical cultures, the IC50 of neuronal activity suggests that neurotoxicity may occur at doses exceeding 330-650mg. In practical terms, users should avoid high doses to prevent potential long-term harm, but standard doses are generally considered safe.
Cardiac Risk:
Some users have reported hypertension, hyperthermia, and tachycardia at elevated doses. Consequently, individuals with pre-existing heart conditions should abstain from 2C-B use. Users should monitor their heart rate and body temperature and respond accordingly. Engaging in strenuous physical activities while on 2C-B is discouraged.
Lethal Dosage:
There is no available data on the LD50 (lethal dose) of 2C-B. However, it is believed to be considerably higher than the effective dose. For reference, Alexander Shulgin reported the consumption of a 100 mg oral dose without apparent harm.
Dependence and Abuse Potential:
2C-B, like other serotonergic psychedelics, is generally regarded as non-addictive with a low potential for abuse.
Tolerance:
Tolerance to the effects of 2C-B does not typically develop immediately after ingestion. Many anecdotal reports indicate that individuals have consumed this substance over consecutive days, redosing numerous times, without an immediate tolerance buildup. Tolerance tends to increase slowly, even under sustained exposure. Notably, 2C-B does not exhibit cross-tolerance with other serotonergic psychedelics, although some users report that other psychedelics may affect 2C-B tolerance. For instance, using 2C-B followed by LSD does not diminish the effects, whereas the reverse sequence results in reduced 2C-B effects. However, this one-way tolerance appears to be an exception, with most individuals experiencing cross-tolerance between 2C-B and other psychedelics.
Dangerous Interactions:
It’s crucial to note that many psychoactive substances, which are relatively safe when used alone, can become dangerous and potentially life-threatening when combined with specific other substances. Here are some known dangerous interactions to be cautious of:
- Lithium: Lithium, commonly prescribed for bipolar disorder, is strongly discouraged when used alongside psychedelics due to the heightened risk of psychosis and seizures.
- Cannabis: Combining cannabis with 2C-B can lead to a potent and unpredictable synergy, significantly increasing the risk of adverse psychological reactions such as anxiety, paranoia, panic attacks, and psychosis. Caution is advised, with users recommended to start with a reduced cannabis dose and take extended breaks between hits to prevent unintentional overdose.
- Stimulants: Stimulants like amphetamine, cocaine, or methylphenidate can interact with psychedelics, potentially elevating the risk of anxiety, paranoia, panic attacks, and thought loops. This combination may also enhance the chances of experiencing mania and psychosis.
- Tramadol: Tramadol is known to lower the seizure threshold, and when combined with psychedelics, it could potentially trigger seizures in susceptible individuals. Caution is strongly advised.
Legal status
Internationally, 2C-B was incorporated into the UN Convention on Psychotropic Substances as a Schedule II substance on March 20, 2001.
Here are the legal statuses of 2C-B in various countries:
- Argentina: 2C-B is classified as a Schedule I controlled substance.
- Australia: In Australia, 2C-B is categorized as a Schedule 9 prohibited substance under the Poisons Standard. This designation means that its manufacture, possession, sale, or use is prohibited by law, except for medical or scientificresearch, analytical, teaching, or training purposes with approval from Commonwealth and State or Territory Health Authorities.
- Austria: 2C-B is illegal to possess, produce, and sell in Austria, falling under the SMG (Suchtmittelgesetz Österreich). It is listed in Schedule V of the Suchtgiftverordnung, which further specifies the SMG regulations.
- Belgium: The possession, production, and sale of 2C-B are illegal in Belgium.
- Brazil: 2C-B is prohibited in Brazil, and it is illegal to possess, produce, and sell it as listed on Portaria SVS/MS nº 344.
- Canada: In Canada, 2C-B is classified as a Schedule III drug.
- Croatia: The possession, production, and sale of 2C-B are illegal in Croatia due to its classification as a 2,5-dimethoxyphenylethanamine.
- Czech Republic: 2C-B is considered a Schedule II drug in the Czech Republic.
- Denmark: 2C-B is classified as a List B controlled substance in Denmark.
- Estonia: 2C-B is listed as a Schedule I drug in Estonia.
- Finland: The possession, production, and sale of 2C-B are illegal in Finland.
- Germany: 2C-B has been controlled under Anlage I BtMG (Narcotics Act, Schedule I) since January 31, 1993.[31] Manufacturing, possessing, importing, exporting, buying, selling, procuring, or dispensing it without a license is illegal.
- Italy: In Italy, 2C-B is categorized as a Schedule I (tabella I) drug.
- Japan: Possession, production, and sale of 2C-B are illegal.
- Latvia: 2C-B is classified as a Schedule I controlled substance in Latvia.
- Luxembourg: 2C-B has been a prohibited substance in Luxembourg since 2001.
- The Netherlands: The possession, production, and sale of 2C-B are illegal.
- Norway: 2C-B is categorized as a Schedule II drug.
- Poland: Since 2015, the possession of 2C-B is illegal in Poland under “Wykaz środków odurzających i substancji psychotropowych.”
- Russia: The possession, production, and sale of 2C-B are illegal.
- Spain: In Spain, 2C-B is classified as a Category 2 drug.
- Sweden: 2C-B is considered a Schedule I drug in Sweden.
- Switzerland: 2C-B is a controlled substance named explicitly under Verzeichnis D.
- Turkey: 2C-B is classified as a drug and is illegal to possess, produce supply, or import in Turkey.
- United Kingdom: In the United Kingdom, 2C-B is classified as a Class A drug, primarily due to the phenethylamine catch-all clause.
- United States: 2C-B is classified as a Schedule I drug.
FAQ
1. What is 2C-B?
2C-B, also known as 2,5-dimethoxy-4-bromophenethylamine, is a synthetic psychedelic substance in the phenethylamine class. It is known for its hallucinogenic and stimulant properties and is chemically similar to mescaline.
2. How is 2C-B typically consumed?
2C-B is commonly taken orally as a tablet, capsule, or powder. It can also be insufflated (snorted) or used rectally, although these routes of administration may have different effects and durations.
3. What are the effects of 2C-B?
The effects of 2C-B can vary depending on the dose and individual sensitivity. Common effects include open and closed-eye visuals, time distortion, euphoria, and altered sensory perception. Some users also report enhanced tactile sensations. It is often described as having a more manageable headspace than other psychedelics like LSD or psilocybin.
4. How long does a 2C-B trip last?
The duration of a 2C-B trip can vary but typically ranges from 4 to 8 hours. The onset of effects is relatively rapid, usually occurring within 20 to 60 minutes after ingestion.
5. Is 2C-B legal?
The legal status of 2C-B varies by country. In many places, including the United States, it is classified as a Schedule I controlled substance, making it illegal to possess, manufacture, or distribute. Always check your local laws and regulations.
6. Is 2C-B safe to use?
The safety of 2C-B use is a topic of ongoing debate. While it generally has a lower risk profile than other psychedelics, adverse reactions such as anxiety, paranoia, and hallucinatory states are possible, especially at higher doses. More research is needed to understand its long-term effects fully.
7. Can 2C-B be used for therapeutic purposes?
There is limited research on the therapeutic potential of 2C-B, but in the 1970s, it was used by some psychotherapists to facilitate therapy sessions due to its empathetic and mild nature. However, it is not currently approved for therapeutic use, and its legal status restricts such applications.
8. Are there any known interactions or risks with 2C-B?
Combining 2C-B with other substances, especially alcohol, stimulants, or cannabis, can increase the risk of adverse reactions. It’s essential to be cautious when mixing substances and consider potential interactions and health risks.
9. Can 2C-B be addictive?
2C-B is generally considered non-addictive with a low potential for abuse. Users are less likely to develop a physical dependence on this substance compared to drugs like opioids or stimulants.
10. Is there a safe dosage for 2C-B?
There is no universally safe dosage for 2C-B, as individual reactions can vary. It is recommended to start with a low dose and gradually increase it if needed, under the guidance of experienced users or professionals. Use harm reduction practices and be aware of your tolerance and sensitivity.
11. Can 2C-B be tested for purity?
Yes, various drug testing kits are available to help identify the presence of 2C-B and assess its purity. Harm reduction organizations and individuals commonly use these kits to verify the content of substances.
12. What should I do in case of a bad trip on 2C-B?
If you or someone you are with experiences a challenging or uncomfortable trip, creating a calm and supportive environment is essential. You can change the setting, engage in grounding techniques, and seek assistance from a trusted friend or professional. It’s crucial to prioritize safety and well-being during a psychedelic experience.
References
1. Pablo Mallaroni, Natasha L. Mason, Johannes T. Reckweg, Riccardo Paci, Sabrina Ritscher, Stefan W. Toennes, Eef L. Theunissen, Kim P.C. Kuypers, Johannes G. Ramaekers (May 2023). “Assessment of the acute effects of 2C-B vs psilocybin on subjective experience, mood, and cognition.” Clinical Pharmacology and Therapeutics. doi:10.1002/cpt.2958.
2. Alexander Shulgin; Ann Shulgin (1991). “#20. 2C-B.” PiHKAL: A Chemical Love Story. United States: Transform Press. ISBN 0963009605. OCLC 1166889264.
3. Shulgin, A. T.; Carter, M. F. (1975). “Centrally active phenethylamines.” Psychopharmacology Communications. 1 (1): 93–98. ISSN 0098-616X. OCLC 924603662. PMID 1223994.
4. “2C-B: Effects.” Erowid. February 12, 1998 [Modified 2015]. Retrieved February 10, 2020.
5. “2C-B (Nexus) Reappears on the Club Drug Scene” (PDF). Information Bulletin. Drug Enforcement Agency (DEA). May 2001. 2001-L0424-002.
6. Strassmann, Rick (1984). “Adverse reactions to psychedelic drugs. A review of the literature.” Journal of Nervous and Mental Disease. 172 (10): 577–595. doi:10.1097/00005053-198410000-00001. ISSN 0022-3018. OCLC 1754691. PMID 6384428.
7. Alexander Shulgin; Ann Shulgin (1991). PiHKAL: A Chemical Love Story. United States: Transform Press. ISBN 0963009605. OCLC 1166889264.
8. David Biello (March 20, 2008). “Self-Experimenters: Psychedelic Chemist Explores the Surreality of Inner Space, One Drug at a Time.” Scientific American. Nature Publishing Group. eISSN 1946-7087. ISSN 0036-8733. Retrieved October 10, 2020.
9. “2C-B (Nexus).” Encyclopedia.com. Retrieved October 10, 2020.
10. “History of Nexus.” Tacethno.com. March 27, 2008. Retrieved May 15, 2012. [dubious – discuss]
11. “Anu” (February 1996) [Modified 2016]. “The Nexus Factor: An Introduction to 2C-B.” Erowid. Retrieved October 10, 2020.
12. Ubulawu Nomathotholo Package (Picture). Erowid. 2002.
13. Moya, P. R.; Berg, K. A.; Gutiérrez-Hernandez, M. A.; Sáez-Briones, P.; Reyes-Parada, M.; Cassels, B. K.; Clarke, W. P. (2007). “Functional Selectivity of Hallucinogenic Phenethylamine and Phenylisopropylamine Derivatives at Human 5-Hydroxytryptamine (5-HT)2A and 5-HT2C Receptors.” Journal of Pharmacology and Experimental Therapeutics. 321 (3): 1054–1061. doi:10.1124/jpet.106.117507. eISSN 1521-0103. ISSN 0022-3565. OCLC 1606914. PMID 17337633.
14. Villalobos, C. A.; Bull, P.; Sáez, P.; Cassels, B. K.; Huidobro‐Toro, J. P. (2004). “4‐Bromo‐2,5‐dimethoxyphenethylamine (2C‐B) and structurally related phenylethylamines are potent 5‐HT2A receptor antagonists in Xenopus laevis oocytes.” British Journal of Pharmacology. 141 (7): 1167–1174. doi:10.1038/sj.bjp.0705722 Freely accessible. eISSN 1476-5381. ISSN 0007-1188. OCLC 01240522. PMC 1574890 Freely accessible. PMID 15006903.
15. Páleníček, T.; Fujáková, M.; Brunovský, M.; Horáček, J.; Gorman, I.; Balíková, M.; Rambousek, L.; Syslová, K.; Kačer, P.; Zach, P.; Bubeníková-Valešová, V.; Tylš, F.; Kubešová, A.; Puskarčíková, J.; Höschl, C. (2013). “Behavioral, neurochemical and pharmaco-EEG profiles of the psychedelic drug 4-bromo-2,5-dimethoxyphenethylamine (2C-B) in rats.” Psychopharmacology. 225: 75–93. doi:10.1007/s00213-012-2797-7. eISSN 1432-2072. ISSN 0033-3158. OCLC 2409222. PMID 22842791.
16. Armstrong, B. D.; Paik, E.; Chhith, S.; Lelievre, V.; Waschek, J. A.; Howard, S. G. (October 26, 2004). “Potentiation of (DL)‐3, 4‐methylenedioxymethamphetamine (MDMA)‐induced toxicity by the serotonin 2A receptor partial agonist d‐lysergic acid diethylamide (LSD), and the protection of same by the serotonin 2A/2C receptor antagonist MDL 11,939.” Neuroscience Research Communications. 35 (2): 83–95. doi:10.1002/nrc.20023. eISSN 1520-6769.
17. Hondebrink, L.; Zwartsen, A.; Westerink, R. H. S. (2018). “Effect fingerprinting of new psychoactive substances (NPS): What can we learn from in vitro data?” Pharmacology & Therapeutics. 182: 193–224. doi:10.1016/j.pharmthera.2017.10.022 Freely accessible. eISSN 1879-016X. ISSN 0163-7258. OCLC 04981366. PMID 29097307.
18. Zwartsen, A.; Hondebrink, L.; Westerink, R. H. S. (2018). “Neurotoxicity screening of new psychoactive substances (NPS): Effects on neuronal activity in rat cortical cultures using microelectrode arrays (MEA).” NeuroToxicology. 66: 87–97. doi:10.1016/j.neuro.2018.03.007. ISSN 0161-813X. OCLC 47153737. PMID 29572046.
19. Papaseit, E.; Farré, M.; Pérez-Mañá, C.; Torrens, M.; Ventura, M.; Pujadas, M.; de la Torre, R.; González, D. (2018). “Acute Pharmacological Effects of 2C-B in Humans: An Observational Study.” Frontiers in Pharmacology. 9: 206. doi:10.3389/fphar.2018.00206 Freely accessible. ISSN 1663-9812. OCLC 1198838203. PMC 5859368 Freely accessible. PMID 29593537.
20. Talaie, H.; Panahandeh, R.; Fayaznouri, M. R.; Asadi, Z.; Abdollahi, M. (2009). “Dose-independent occurrence of seizure with tramadol.” Journal of Medical Toxicology. 5 (2): 63–67. doi:10.1007/BF03161089. ISSN 1556-9039.
21. “Inclusion of 4-bromo-2,5-dimethoxyphenethylamine (2C-B) in Schedule II of the Convention on Psychotropic Substances of 1971.” United Nations Office on Drugs and Crime (UNODC). March 20, 2001. CND Dec.44/1. Retrieved December 10, 2019.
22. “Decreto 299/2010: Actualización de la lista de estupefacientes y demás sustancias químicas que deberán ser incluidas en los alcances de la Ley Nº 23.737” (PDF) (in Spanish). February 3, 2010. Retrieved December 10, 2019.
23. “Poisons Standard October 2015.” Federal Register of Legislation.
24. “Bundesrecht konsolidiert: Gesamte Rechtsvorschrift für Suchtgiftverordnung” (in German). May 18, 2020. Retrieved January 10, 2021.
25. “RESOLUÇÃO DA DIRETORIA COLEGIADA – RDC N° 130, DE 2 DE DEZEMBRO DE 2016” (in Portuguese). Agência Nacional de Vigilância Sanitária (ANVISA) [Brazilian Health Regulatory Agency (ANVISA)]. December 5, 2016.
26. “Schedule III.” Controlled Drugs and Substances Act (CDSA). Isomer Design. Retrieved October 10, 2020.
27. “Popis: Droga, psihotropnih tvari i biljaka iz kojih se može dobiti droga te tvari koje se mogu uporabiti za izradu droga” (in Croatian). January 29, 2016. ISSN 1331-7725. OCLC 299165185.
28. https://www.zakonyprolidi.cz/cs/2013-463#f5150333
29. “Bekendtgørelse om euforiserende stoffer” (in Danish). May 31, 2011. BEK Nr. 557. Retrieved December 10, 2019.
30. “Betäubungsmittelgesetz (BtMG) Anlage I” [Narcotics Act (BtMG) Schedule I] (in German). Bundesamt für Justiz [Federal Office of Justice]. Retrieved December 10, 2019.
31. “Vierte Verordnung zur Änderung betäubungsmittelrechtlicher Vorschriften” (PDF). Bundesgesetzblatt Jahrgang 1992 Teil I Nr. 61 (in German). Bundesanzeiger Verlag [Federal Gazette] (published December 31, 1992). December 23, 1992. p. 1058. eISSN 0344-7634.
32. “Betäubungsmittelgesetz (BtMG) § 29” [Narcotics Act (BtMG) § 29] (in German). Bundesamt für Justiz [Federal Office of Justice]. Retrieved December 10, 2019.
33. “Tabella I” (PDF) (in Italian). Ministero della Salute [Ministry of Health]. p. 8. Retrieved January 7, 2020.
34. “Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām vielām un prekursoriem” (in Latvian). VSIA Latvijas Vēstnesis. November 10, 2005. Retrieved January 1, 2020.
35. Règlement grand-ducal du 14 décembre 2001 modifiant l’annexe du règlement grand-ducal modifié du 4 mars 1974 concernant certaines substances toxiques. | http://legilux.public.lu/eli/etat/leg/rgd/2001/12/14/n10/jo
36. https://pl.wikipedia.org/wiki/2C-B#Stan_prawny_w_Polsce
37. “Föreskrifter om ändring i Läkemedelsverkets föreskrifter (LVFS 1997:12) om förteckningar över narkotika” (PDF). Läkemedelsverkets författningssamling (LVFS) (in Swedish). Läkemedelsverket [Swedish Medical Products Agency] (published December 8, 2009). November 25, 2009. p. 1. ISSN 1101-5225. OCLC 185277860. LVFS 2009:22. Archived from the original (PDF) on September 16, 2018.
38. “Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien” (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.
39. “Bakanlar Kurulu Kararı – Karar Sayısı: 2011/1310”. Resmî Gazete, Sayı: 27845 (in Turkish). Başbakanlık Mevzuatı Geliştirme ve Yayın Genel Müdürlüğü [General Directorate of Legislation Development and Publication] (published February 13, 2011). January 7, 2011.
40. “Part I: Class A Drugs.” “Misuse of Drugs Act 1971.” UK Government. Retrieved January 7, 2020.
41. “Part 1308: Schedules of Controlled Substances: §1308.11 Schedule I.” Title 21 Code of Federal Regulations. Diversion Control Division. Retrieved November 5, 2019.