The market situation for 3-FA, a designer drug categorized as a research chemical, is complex and constantly evolving. 3-FA, also known as 3-Fluoroamphetamine, has garnered attention in recent years within online communities. Here is an overview of the market:
Online Presence: 3-FA is primarily bought and sold through online vendors and marketplaces that specialize in research chemicals. These platforms offer a wide range of designer drugs, making them accessible to a global customer base.
Seller Diversity: Numerous sellers and vendors are involved in the trade of 3-FA, contributing to a competitive market. Buyers can choose from various sources, each offering different product quality and pricing.
Designer Drug Status: 3-FA falls into the category of designer drugs, meaning it is chemically engineered to mimic the effects of other controlled substances. This classification allows vendors to operate in a legal gray area, as regulations often lag behind the emergence of new compounds.
For Sale Worldwide: 3-FA is available for sale worldwide, though its legality varies significantly from one jurisdiction to another. Buyers should be aware of their local regulations regarding the purchase and possession of such substances.
Market Fluctuations: The market for research chemicals like 3-FA is subject to rapid changes due to legal crackdowns and shifts in consumer preferences. Sellers may frequently alter their product offerings to adapt to evolving regulations.
Contents
- 1 Summary
- 2 Chemistry
- 3 Pharmacology
- 4 Subjective effects
- 5 Toxicity
- 6 Legal status
- 7 FAQ
- 7.1 1. What is 3-FA?
- 7.2 2. What are the common names for 3-FA?
- 7.3 3. How is 3-FA typically used?
- 7.4 4. Is 3-FA legal?
- 7.5 5. What are the effects of 3-FA?
- 7.6 6. Is 3-FA safe to use?
- 7.7 7. Can 3-FA be used recreationally?
- 7.8 8. Are there any known health risks associated with 3-FA?
- 7.9 9. How should 3-FA be stored and handled in a research setting?
- 7.10 10. Can I buy 3-FA online?
- 7.11 11. Is 3-FA the same as other amphetamines or designer drugs?
- 7.12 12. Can I mix 3-FA with other substances?
- 7.13 13. How can I stay safe when researching 3-FA?
- 8 References
Summary
3-Fluoroamphetamine (3-FA) is a synthetic amphetamine derivative, incorporating a fluorine substitution on the amphetamine ring structure. This compound is renowned for its potent classical stimulant properties, often being described as nearly as powerful as methamphetamine[^1^]. It belongs to a family of designer amphetamines that feature fluorine substitutions, including 2-FA, 2-FMA, 3-FEA, and 4-FA. These compounds are notable for their ability to induce euphoria and stimulation, gaining popularity as research chemical alternatives to traditional street stimulants.
It’s important to note that 3-FA is seldom encountered in street drug markets, with limited availability. Instead, it occasionally appears within the grey market as a research chemical, primarily distributed through online vendors. This little street presence, coupled with its classification as a research chemical, places it in a unique and evolving niche within the broader stimulant landscape. Researchers and individuals interested in these substances should exercise caution and stay informed about changing legal and safety considerations.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1626-71-7 |
|---|---|
| PubChem CID | 121501 |
| ChemSpider | 108417 |
| UNII | 4KKW6IBP5X |
| CompTox Dashboard (EPA) | DTXSID40936718 |
| Chemical and physical data | |
| Formula | C9H12FN |
| Molar mass | 153.200 g·mol−1 |
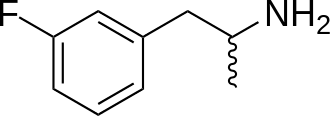
Chemistry
3-Fluoroamphetamine, often referred to as 3-FA, belongs to the amphetamine class of synthetic molecules. Amphetamines share a standard phenethylamine structure, characterized by a phenyl ring linked to an amino (NH2) group through an ethyl chain, and typically, they possess an additional methyl substitution at the Rα position (meaning they are alpha-methylated phenethylamines). Notably, 3-FA lacks the methyl group attached to the terminal amine RN of the amphetamine core, making it structurally and functionally akin to amphetamine itself. In essence, 3-FA can be regarded as the 3-fluorinated counterpart of amphetamine.
Pharmacology
While formal research on 3-FA has not reached the same depth as traditional amphetamines, it’s reasonable to hypothesize that, like other substituted amphetamines with similar structural modifications (with the notable exception of 4-FA), it likely primarily functions as a dopamine and norepinephrine-releasing agent. Additionally, it may exhibit moderate selectivity for serotonin. This means that 3-FA is likely to enhance the levels of dopamine and norepinephrine neurotransmitters within the brain. It achieves this by binding to and partially obstructing the transporter proteins responsible for clearing and reuptaking these molecules from the synaptic cleft, where they are released during neural signaling. Consequently, dopamine and norepinephrine can accumulate in the brain to levels beyond what the body naturally produces. This accumulation is known to have stimulating, motivating, and euphoric effects in humans.
Subjective effects
3-FA is acknowledged as a potent and multifaceted stimulant, distinguishing itself from other substances in its class, such as 4-FA, by offering mild entactogenic qualities. However, it needs to exhibit the productivity and focus-enhancing attributes commonly associated with 2-FA or 2-FMA, which has somewhat limited its appeal.
Please note that the effects detailed below are drawn from the Subjective Effect Index (SEI), a compilation of anecdotal user experiences and analyses by contributors to PsychonautWiki. Consequently, these effects should be approached with a degree of skepticism.
It’s crucial to understand that these effects may not manifest predictably or consistently, and higher doses are more likely to encompass the full range of products. Additionally, escalating doses increase the likelihood of adverse consequences, including the potential for addiction, severe harm, or even fatality ☠.
Physical:
- Stimulation
- Tactile enhancement (typically more pronounced at higher doses)
- Physical euphoria
- Dehydration
- Appetite suppression
- Increased heart rate
- Increased perspiration
- Teeth grinding (comparatively less intense than MDMA or 2-FMA)
After:
- The post-stimulant phase, often termed a “comedown,” is typically characterized by negative and uncomfortable sensations due to neurotransmitter depletion. It may include:
- Anxiety
- Cognitive fatigue
- Depression
- Irritability
- Suppressed motivation
- Slowed thought processes
- Wakefulness
Cognitive:
- Cognitive euphoria
- Accelerated thought processes
- Enhanced focus
- Anxiety alleviation
- Heightened wakefulness
- Improved analytical abilities
- Increased motivation
- Compulsive redosing
- Enhanced music appreciation (more evident at higher doses, as lower to medium quantities tend to be productivity-focused)
- Time distortion, involving the perception of time passing faster than usual when sober
Toxicity
Below is a revised list of potentially dangerous interactions with substances, although it may not cover all possible interactions. Always conduct thorough independent research (e.g., using Google, DuckDuckGo, PubMed) to ensure the safety of combining two or more substances. Some of these interactions have been sourced from TripSit:
- Alcohol: Combining alcohol with stimulants is risky because it diminishes the sedative effects of alcohol that typically help gauge drunkenness. This can lead to excessive drinking, reduced inhibitions, and an increased risk of liver damage and dehydration. Stimulants can also mask the usual signs of alcohol intoxication, potentially allowing one to consume dangerous amounts.
- GHB/GBL: Stimulants increase respiration rates, potentially allowing for higher doses of sedatives. If the stimulant wears off before the depressant effects of GHB/GBL, it may lead to respiratory arrest.
- Opioids: Stimulants can also increase respiration rates, potentially permitting higher doses of opioids. If the stimulant’s effects wear off before the opioids, it could lead to respiratory arrest.
- Cocaine: Combining amphetamines with cocaine can lead to cardiac effects due to interactions with serotonin receptors. This combination may increase the risk of heart issues, including valvulopathy, hypertension, and syncope.
- Cannabis: Stimulants can raise anxiety levels and increase the risk of thought loops and paranoia when combined with cannabis, potentially leading to negative experiences.
- Caffeine: Combining stimulants like caffeine and other stimulants may be unnecessary and can strain the heart, potentially causing anxiety and physical discomfort.
- Tramadol: Both tramadol and stimulants increase the risk of seizures when combined.
- DXM: Both substances raise heart rate, potentially leading to panic attacks and more severe heart issues in extreme cases.
- Ketamine: Combining amphetamines and ketamine may result in psychoses resembling schizophrenia, mainly due to interactions in the dopaminergic pathways. This combination can lead to thought disorders and positive symptoms.
- PCP: Combining stimulants with PCP can increase the risk of tachycardia, hypertension, and manic states.
- Methoxetamine: Mixing stimulants with methoxetamine can increase the risk of tachycardia, hypertension, and manic states.
- Psychedelics (e.g., LSD, mescaline, psilocybin): Combining stimulants with psychedelics can heighten the risk of anxiety, paranoia, and thought loops.
- 25x-NBOMe: The stimulation from amphetamines and NBOMes can result in tachycardia, hypertension, vasoconstriction, and, in extreme cases, heart failure when combined. The anxiogenic effects of stimulants can also exacerbate thought loops.
- 2C-T-x and 5-MeO-xxT: These substances may have mild MAOI properties, potentially increasing the risk of hypertensive crisis when combined with amphetamines.
- DOx: Interaction risks exist with DOx compounds, although specific details may vary.
- aMT: aMT has MAOI properties, which could interact unfavorably with amphetamines.
- MAOIs: MAO-B inhibitors can enhance the potency and duration of phenethylamines unpredictably, while MAO-A inhibitors combined with amphetamines can lead to hypertensive crises.
Legal status
3-FA currently resides in a legal gray area globally, signifying that its regulation lacks clarity, and it is not explicitly designated as illegal (or “scheduled”) in any country. Nevertheless, individuals could potentially face charges related to their possession under specific circumstances, including analog laws and instances involving intent to sell or consume.
Here’s the status of 3-FA in select countries:
- China: As of October 2015, 3-FA is a controlled substance in China.
- Germany: 3-FA falls under the New Psychoactive Substances Act (NpSG) as of November 26, 2016. Production, import with the intent to market, administration to others, and trading are punishable offenses. Possession is illegal but not penalized.
- New Zealand: In New Zealand, 3-FA is categorized as a Schedule 3 controlled substance due to its status as an amphetamine analog.
- Switzerland: 3-FA is explicitly listed as a controlled substance under Verzeichnis E in Switzerland.
- Turkey: In Turkey, 3-FA is classified as a drug and is illegal to possess, produce, supply, or import.
- United Kingdom: 3-FA is classified as a Class A drug in the United Kingdom, primarily because of the amphetamine analog clause within the Misuse of Drugs Act 1971.
- United States: In the United States, 3-FA may potentially be considered an analog of amphetamine, subject to the Federal Analogue Act. This act allows chemicals that are “substantially similar” to illegal drugs in Schedule I or II to be treated as if they are also in Schedule I or II, but only when intended for human consumption.
FAQ
1. What is 3-FA?
- 3-FA, or 3-Fluoroamphetamine, is a synthetic compound that belongs to the amphetamine class. It shares structural similarities with amphetamine and is known for its stimulant effects.
2. What are the common names for 3-FA?
- Some common names for 3-FA include “3-Fluoroamphetamine” and “3-Flo.”
3. How is 3-FA typically used?
- 3-FA is often used as a research chemical in scientific studies to investigate its properties and effects. It is not intended for human consumption.
4. Is 3-FA legal?
- The legal status of 3-FA varies by country. In some places, it may be considered a controlled substance, while in others, it may exist in a legal gray area. Always check local laws and regulations before obtaining or using 3-FA.
5. What are the effects of 3-FA?
- The effects of 3-FA are not well-documented in scientific literature due to limited research. However, it is believed to have stimulant properties similar to amphetamine, potentially leading to increased alertness, energy, and focus.
6. Is 3-FA safe to use?
- The safety of 3-FA has yet to be thoroughly studied, and its use may carry risks. It is essential to exercise caution and follow responsible research practices when working with this compound.
7. Can 3-FA be used recreationally?
- While some individuals may use 3-FA recreationally, seeking its stimulant effects, it is essential to remember that its legality varies, and its safety profile is not well-established. Using it for recreational purposes can be risky.
8. Are there any known health risks associated with 3-FA?
- Limited research means that potential health risks associated with 3-FA use are not well-documented. However, as a stimulant, it may carry risks like increased heart rate, blood pressure, and potential addiction.
9. How should 3-FA be stored and handled in a research setting?
- In a research setting, 3-FA should be stored in a controlled environment, following safety guidelines for handling potentially hazardous chemicals. Always wear appropriate protective gear and keep it securely to prevent unauthorized access.
10. Can I buy 3-FA online?
- The availability of 3-FA for purchase online may vary by region and local regulations. It is crucial to research and verify the legality of obtaining 3-FA in your area before attempting to purchase it.
11. Is 3-FA the same as other amphetamines or designer drugs?
- 3-FA is a distinct compound within the amphetamine class and should not be confused with other substances, even though they may share some structural similarities.
12. Can I mix 3-FA with other substances?
- Combining 3-FA with other substances can be dangerous and is not recommended. Always conduct thorough research and consult with experts before considering any combinations.
13. How can I stay safe when researching 3-FA?
- To stay safe when researching 3-FA, follow responsible research practices, ensure compliance with local laws, and prioritize safety protocols in a controlled laboratory environment.
References
1. Negus, S. S., Mello, N. K., Blough, B. E., Baumann, M. H., Rothman, R. B. (February 2007). “Monoamine Releasers with Varying Selectivity for Dopamine/Norepinephrine versus Serotonin Release as Candidate “Agonist” Medications for Cocaine Dependence: Studies in Assays of Cocaine Discrimination and Cocaine Self-Administration in Rhesus Monkeys.” Journal of Pharmacology and Experimental Therapeutics. 320 (2): 627–636. doi:10.1124/jpet.106.107383. ISSN 0022-3565.
2. Rösner, P., Quednow, B., Girreser, U., Junge, T. (10 March 2005). “Isomeric fluoro-methoxy-phenyl alkylamines: a new series of controlled-substance analogs (designer drugs).” Forensic Science International. 148 (2–3): 143–156. doi:10.1016/j.forsciint.2004.05.003. ISSN 0379-0738.
3. Camilleri, A., Johnston, M. R., Brennan, M., Davis, S., Caldicott, D. G. E. (15 April 2010). “Chemical analysis of four capsules containing the controlled substance analogs 4-methylmethcathinone, 2-fluoromethamphetamine, alpha-phthalimidopropiophenone and N-ethylcathinone”. Forensic Science International. 197 (1–3): 59–66. doi:10.1016/j.forsciint.2009.12.048. ISSN 1872-6283.
4. Smart, B. E. (June 1 2001). “Fluorine substituent effects (on bioactivity).” Journal of Fluorine Chemistry. 109 (1): 3–11. doi:10.1016/S0022-1139(01)00375-X. ISSN 0022-1139.
5. Shoptaw, S. J., Kao, U., Ling, W. (January 21, 2009). Cochrane Drugs and Alcohol Group, ed. “Treatment for amphetamine psychosis.” Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD003026.pub3. ISSN 1465-1858.
6. Hofmann, F. G. (1983). A handbook on drug and alcohol abuse: the biomedical aspects (2nd ed ed.). Oxford University Press. ISBN 9780195030563.
7. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021303s026lbl.pdf
8. Greenwald, M. K., Lundahl, L. H., Steinmiller, C. L. (December 2010). “Sustained Release d-Amphetamine Reduces Cocaine but not ‘Speedball’-Seeking in Buprenorphine-Maintained Volunteers: A Test of Dual-Agonist Pharmacotherapy for Cocaine/Heroin Polydrug Abusers.” Neuropsychopharmacology. 35 (13): 2624–2637. doi:10.1038/npp.2010.175. ISSN 0893-133X.
9. Siciliano, C. A., Saha, K., Calipari, E. S., Fordahl, S. C., Chen, R., Khoshbouei, H., Jones, S. R. (January 10, 2018). “Amphetamine Reverses Escalated Cocaine Intake via Restoration of Dopamine Transporter Conformation.” The Journal of Neuroscience. 38 (2): 484–497. doi:10.1523/JNEUROSCI.2604-17.2017. ISSN 0270-6474.
10. Krystal, J. H., Perry, E. B., Gueorguieva, R., Belger, A., Madonick, S. H., Abi-Dargham, A., Cooper, T. B., MacDougall, L., Abi-Saab, W., D’Souza, D. C. (September 1, 2005). “Comparative and Interactive Human Psychopharmacologic Effects of Ketamine and Amphetamine: Implications for Glutamatergic and Dopaminergic Model Psychoses and Cognitive Function.” Archives of General Psychiatry. 62 (9): 985. doi:10.1001/archpsyc.62.9.985. ISSN 0003-990X.
11. “关于印发《非药用类麻醉药品和精神药品列管办法》的通知” (in Chinese). China Food and Drug Administration. September 27, 2015. Retrieved October 1, 2015.
12. “Anlage NpSG” (in German). Bundesministerium der Justiz und für Verbraucherschutz. Retrieved December 19, 2019.
13. “Gesetz zur Bekämpfung der Verbreitung neuer psychoaktiver Stoffe” (PDF) (in German). Bundesanzeiger Verlag. Retrieved December 19, 2019.
14. “§ 4 NpSG” (in German). Bundesministerium der Justiz und für Verbraucherschutz. Retrieved December 19, 2019.
15. Misuse of Drugs Act 1975 No 116 (as of July 1, 2022), Public Act – New Zealand Legislation.
16. “Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien” (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.
17. Başbakanlık Mevzuatı Geliştirme ve Yayın Genel Müdürlüğü.
18. https://resmigazete.gov.tr/eskiler/2014/01/20140125-3-1.pdf
19. Misuse of Drugs Act 1971.