Contents
- 1 Summary
- 2 Chemistry
- 3 Pharmacology
- 4 Legality
- 5 FAQ
- 5.1 1. What is 3-Fluorophenmetrazine (3-FPM)?
- 5.2 2. Is 3-FPM legal in the United States?
- 5.3 3. When did 3-FPM become illegal in Virginia, USA?
- 5.4 4. Are other positional isomers of 3-FPM illegal in Virginia or at the federal level in the USA?
- 5.5 5. Is 3-FPM illegal in Sweden?
- 5.6 6. Is 3-FPM legal in Switzerland?
- 6 References
Summary
3-Fluorophenmetrazine, commonly referred to as 3-FPM and also known as 3-FPH and PAL-593, is a stimulant with its foundation in phenylmorpholine. This chemical compound serves as a fluorinated derivative of phenmetrazine and has been distributed via online sources under the guise of a designer drug.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1350768-28-3 HCl: 1803562-83-5 |
|---|---|
| PubChem CID | 54673723HCl: 75481405 |
| ChemSpider | 37509994HCl: 34212559 |
| UNII | BEV6RF569G |
| Chemical and physical data | |
| Formula | C11H14FNO |
| Molar mass | 195.237 g·mol−1 |
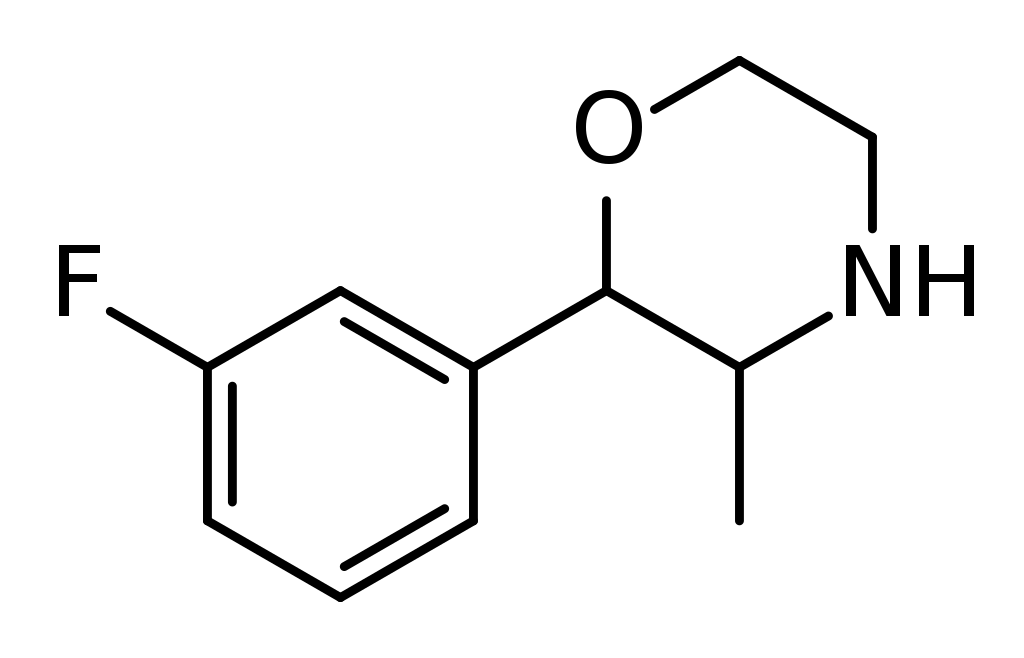
Chemistry
3-Fluorophenmetrazine, belonging to the morpholine class, is a fluorinated counterpart of phenmetrazine, a stimulant.
It’s worth noting that 3-Fluorophenmetrazine is a regioisomer, differing in chemical structure, from both 2-fluorophenmetrazine and 4-fluorophenmetrazine.
Pharmacology
3-FPM exhibits its actions as a norepinephrine–dopamine releasing agent, with EC50 values of 30 nM and 43 nM, respectively, indicating its potency in releasing these neurotransmitters. However, it displays minimal effectiveness in promoting serotonin release, as evidenced by an EC50 value of 2558 nM.
Additionally, 3-FPM hinders the uptake facilitated by dopamine transporters and norepinephrine transporters in HEK293 cells, showing potencies similar to cocaine, with IC50 values less than 2.5 μM. However, its impact on serotonin transporters is less pronounced, with IC50 values exceeding 80 μM.
At sufficiently high doses, 3-FPM can reverse the action of monoamine transporters, particularly those associated with the catecholamines dopamine and norepinephrine, and, to a lesser extent, serotonin transporters. This reversal leads to the release of these neurotransmitters from the cytosol into the extracellular space, where they become active.
Studies into its metabolic pathway have revealed processes such as N-oxidation, aryl hydroxylation followed by O-methylation, alkyl hydroxylation, oxidation, and the degradation of the ethyl-bridge, resulting in the formation of the O/N-bis-dealkylated metabolite. These processes can occur in combination with further glucuronidation or sulfation reactions.
Legality
In the United States, 3-fluorophenmetrazine is not explicitly prohibited at the federal level. However, it could potentially fall under the federal Analogue Act if it is intended for human consumption and is considered a structural analog of the Schedule II drug Phenmetrazine.
On November 16, 2016, 3-fluorophenmetrazine was banned in the state of Virginia. As of 2019, it is classified as a Schedule I substance in Virginia. Similarly, the positional isomers of 3-fluorophenmetrazine, such as 2-fluorophenmetrazine and 4-fluorophenmetrazine, are also illegal under Virginia state law but not under federal law.
Sweden’s public health agency proposed the classification of 3-Fluorophenmetrazine as an illegal narcotic on June 1, 2015. It was officially classified as such on October 15, 2015.
Furthermore, 3-fluorophenmetrazine has been illegal in Switzerland since December 2015.
FAQ
1. What is 3-Fluorophenmetrazine (3-FPM)?
- 3-Fluorophenmetrazine, often referred to as 3-FPM, is a chemical compound that belongs to the phenylmorpholine class. It is known for its stimulant properties and is considered a derivative of Phenmetrazine.
2. Is 3-FPM legal in the United States?
- At the federal level, 3-FPM is not explicitly illegal. However, it may be subject to regulation under the federal Analogue Act if it is intended for human consumption and considered a structural analog of the Schedule II drug Phenmetrazine.
3. When did 3-FPM become illegal in Virginia, USA?
- 3-FPM was classified as an illegal substance in the state of Virginia on November 16, 2016. As of 2019, it is categorized as a Schedule I substance in Virginia.
4. Are other positional isomers of 3-FPM illegal in Virginia or at the federal level in the USA?
- Yes, other positional isomers of 3-FPM, such as 2-fluorophenmetrazine and 4-fluorophenmetrazine, are also illegal under Virginia state law. However, they are not prohibited under federal law.
5. Is 3-FPM illegal in Sweden?
- Yes, Sweden’s public health agency proposed classifying 3-fluorophenmetrazine as an illegal narcotic on June 1, 2015. It was officially classified as such on October 15, 2015.
6. Is 3-FPM legal in Switzerland?
- No, 3-Fluorophenmetrazine is illegal in Switzerland as of December 2015.
Please note that legal status and regulations regarding 3-Fluorophenmetrazine may vary by country and region, and it is important to be aware of and comply with local laws and regulations regarding its use and possession. Additionally, its use should always be approached with caution due to its potential health risks and unknown long-term effects.
References
- McLaughlin G, Morris N, Kavanagh PV, Dowling G, Power JD, Twamley B, et al. (March 2017). “Test purchase, synthesis and characterization of 3-fluorophenmetrazine (3-FPM) and differentiation from its ortho- and para-substituted isomers” (PDF). Drug Testing and Analysis. 9 (3): 369–377. doi:10.1002/dta.1945. PMID 26810957. S2CID 205762700.
- Bäckberg M, Westerbergh J, Beck O, Helander A (November 2016). “Adverse events related to the new psychoactive substance 3-fluorophenmetrazine – results from the Swedish STRIDA project”. Clinical Toxicology. 54 (9): 819–825. doi:10.1080/15563650.2016.1211288. PMID 27491700. S2CID 26118285.
- Bruce E. Blough; Richard Rothman; Antonio Landavazo; Kevin M. Page; Ann Marie Decker (8 August 2013). “US Patent 20130203752 A1 – Phenylmorpholines and analogues thereof”.
- Mayer FP, Burchardt NV, Decker AM, Partilla JS, Li Y, McLaughlin G, et al. (May 2018). “Fluorinated phenmetrazine “legal highs” act as substrates for high-affinity monoamine transporters of the SLC6 family”. Neuropharmacology. 134 (Pt A): 149–157. doi:10.1016/j.neuropharm.2017.10.006. PMC 7294773. PMID 28988906.
- Mardal M, Miserez B, Bade R, Portolés T, Bischoff M, Hernández F, Meyer MR (September 2016). “3-Fluorophenmetrazine, a fluorinated analogue of phenmetrazine: Studies on in vivo metabolism in rat and human, in vitro metabolism in human CYP isoenzymes and microbial biotransformation in Pseudomonas Putida and wastewater using GC and LC coupled to (HR)-MS techniques”. Journal of Pharmaceutical and Biomedical Analysis. 128: 485–495. doi:10.1016/j.jpba.2016.06.011. PMID 27372653.
- “VA.R. Doc. No. R17-4746”. 17 December 2018.
- “§ 54.1-3446. Schedule I.” Retrieved 8 September 2023.
- “21 U.S. Code § 812 – Schedules of controlled substances”. 8 September 2023.
- “23 nya ämnen kan klassas som narkotika eller hälsofarlig vara” (in Swedish). Folkhälsomyndigheten. 1 June 2015.
- “Nya substanser klassas som narkotika eller hälsofarlig vara” (in Swedish). Folkhälsomyndigheten. 18 August 2015.
- “Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien” (in German). Der Bundesrat.