Contents
Summary
Viloxazine, available under the brand name Qelbree and formerly known as Vivalan and by other trade names, is a medication classified as a selective norepinephrine reuptake inhibitor (NRI). It is employed in the treatment of attention deficit hyperactivity disorder (ADHD) in both children and adults. Interestingly, Viloxazine had a prior history of almost three decades as an antidepressant for addressing depression before its discontinuation. Subsequently, it found a new purpose as a treatment for ADHD.
Viloxazine is administered orally, with its usage varying between an immediate-release form when employed as an antidepressant and an extended-release form for the treatment of ADHD.
As with any medication, there are potential side effects associated with Viloxazine. These may include insomnia, headaches, drowsiness, fatigue, nausea, vomiting, decreased appetite, dry mouth, constipation, irritability, increased heart rate, and elevated blood pressure. It is important to note that while rare, Viloxazine may prompt thoughts of self-harm and behaviors in some individuals. Additionally, it has the potential to trigger mania or hypomania in individuals with bipolar disorder.
Regarding its pharmacological action, Viloxazine functions as a selective Norepinephrine Reuptake Inhibitor (NRI). The immediate-release form of the medication boasts an elimination half-life of approximately 2.5 hours, whereas the extended-release form has a half-life of approximately 7 hours.
The history of Viloxazine dates back to its initial description in 1972, and it was introduced to the European market as an antidepressant in 1974. Notably, it was not available in the United States during this period.[10] In 2002, Viloxazine was discontinued for commercial reasons, only to be repurposed for ADHD treatment and reintroduced in the United States in April 2021. Importantly, Viloxazine is considered a non-stimulant medication, and it lacks known potential for misuse or abuse. It is not classified as a controlled substance.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 46817-91-8 HCl: 35604-67-2 |
|---|---|
| PubChem CID | 5666 |
| DrugBank | DB09185 HCl: DBSALT001262 |
| ChemSpider | 5464 HCl: 64514 |
| UNII | 5I5Y2789ZFHCl: OQW30I1332 |
| KEGG | D08673 HCl: D02572 |
| ChEBI | CHEBI:94405 |
| ChEMBL | ChEMBL306700 HCl: ChEMBL2106483 |
| CompTox Dashboard (EPA) | DTXSID6057900 |
| ECHA InfoCard | 100.051.148 |
| Chemical and physical data | |
| Formula | C13H19NO3 |
| Molar mass | 237.299 g·mol−1 |
Medical uses
Viloxazine is approved for the management of Attention Deficit Hyperactivity Disorder (ADHD) in various age groups, including children aged 6 to 12 years, adolescents aged 13 to 17 years, and adults.
In a phase 3 regulatory randomized controlled trial assessing the effectiveness of viloxazine in treating ADHD among adults, the scores on the Attention-Deficit/Hyperactivity Disorder Investigator Symptom Rating Scale (AISRS) demonstrated notable improvement. With viloxazine therapy, these scores decreased from an initial 38.5 points to 23.0 points post-treatment, reflecting a substantial reduction of 40%. In the placebo group, the scores decreased from 37.6 points at the beginning to 25.9 points, marking a 31% decrease. This resulted in a placebo-subtracted score difference (the difference between the drug and placebo effects) of -3.7 points, representing a 9% improvement in scores attributed to the effects of viloxazine.
Previously Employed for Depression
Viloxazine has a history as an antidepressant, where it was utilized in the treatment of major depressive disorder. It was deemed effective in managing depression, ranging from mild to severe cases, whether with or without co-occurring symptoms. Typically, the prescribed dosage for depression varied between 100 to 400 mg daily, distributed over two to three doses per day.
Forms Available
For ADHD treatment, viloxazine is offered in the form of extended-release capsules, with options including 100 mg, 150 mg, and 200 mg strengths. These capsules can also be opened and their contents sprinkled into food, simplifying administration.
Side effects
Adverse effects encompassed a range of symptoms, including nausea, vomiting, sleep disturbances such as insomnia, reduced appetite, elevated erythrocyte sedimentation rate, abnormalities in EKG and EEG readings, epigastric discomfort, diarrhea, constipation, dizziness or vertigo, orthostatic hypotension, swelling of the lower extremities, difficulty in speech (dysarthria), tremors, heightened psychomotor activity, mental confusion, inappropriate secretion of antidiuretic hormone, heightened transaminase levels, the occurrence of seizures (although a rare event globally, and most animal studies and clinical trials, including those involving epilepsy patients, suggested potential anticonvulsant properties, hence not considered a complete contraindication in epilepsy), and an increase in libido.
Interactions
Viloxazine led to a notable average elevation of 37% in plasma concentrations of phenytoin. Additionally, it was associated with a substantial increase in plasma levels of theophylline while concurrently reducing its clearance from the body. This occasionally led to unintentional theophylline overdoses.
Pharmacology
Pharmacodynamics
Viloxazine functions as a selective norepinephrine reuptake inhibitor, and this mechanism is believed to underlie its therapeutic effectiveness in addressing conditions like ADHD and depression. Specifically, viloxazine displays binding affinities (KD) at human monoamine transporters, with values of 155 to 630 nM for the norepinephrine transporter (NET), 17,300 nM for the serotonin transporter (SERT), and >100,000 nM for the dopamine transporter (DAT). Importantly, viloxazine exhibits minimal affinity for various assessed receptors, including serotonin 5-HT1A and 5-HT2A receptors, dopamine D2 receptors, α1- and α2-adrenergic receptors, histamine H1 receptors, and muscarinic acetylcholine receptors, all of which demonstrate affinities exceeding 10,000 nM.
More recent research suggests that the pharmacodynamics of viloxazine may possess greater complexity than initially thought. In 2020, it was discovered that viloxazine has a significant affinity for serotonin 5-HT2B and 5-HT2C receptors (Ki = 3,900 nM and 6,400 nM) and functions as an antagonist and agonist at these receptors, respectively. It also exhibits weak antagonistic activity at serotonin 5-HT7 receptors and α1B- and β2-adrenergic receptors. While these actions are relatively weak, they may play a role in its effects and potentially contribute to its therapeutic efficacy in treating ADHD.
Pharmacokinetics
Absorption
The bioavailability of extended-release viloxazine, in comparison to an immediate-release formulation, stands at approximately 88%. Peak levels and the area-under-the-curve (AUC) of extended-release viloxazine exhibit proportionality within a dosage range of 100 to 400 mg when administered once daily. Following a single 200 mg dose, the time to reach peak levels is 5 hours, with a range of 3 to 9 hours. The presence of a high-fat meal slightly reduces viloxazine levels and delays the time to peak by around 2 hours. Steady-state levels of viloxazine are achieved after two days of once-daily administration, with no accumulation observed. In children aged 6 to 11 years, viloxazine levels are approximately 40 to 50% higher compared to children aged 12 to 17 years.
Distribution
Viloxazine exhibits a plasma protein binding capacity of 76 to 82% within a concentration range of 0.5 to 10 μg/mL.
Metabolism
The primary metabolic pathways for viloxazine involve the cytochrome P450 enzyme CYP2D6 and the UDP-glucuronosyltransferases UGT1A9 and UGT2B15. The major metabolite of viloxazine is 5-hydroxyviloxazine glucuronide. CYP2D6 poor metabolizers tend to have slightly higher viloxazine levels compared to CYP2D6 extensive metabolizers.
Elimination
Viloxazine is predominantly eliminated via renal excretion, with approximately 90% of the administered dose excreted in urine within 24 hours and less than 1% recovered in feces.
The elimination half-life of immediate-release viloxazine ranges from 2 to 5 hours (with the most reliable studies suggesting 2–3 hours), while the half-life of extended-release viloxazine is approximately 7.02 ± 4.74 hours.
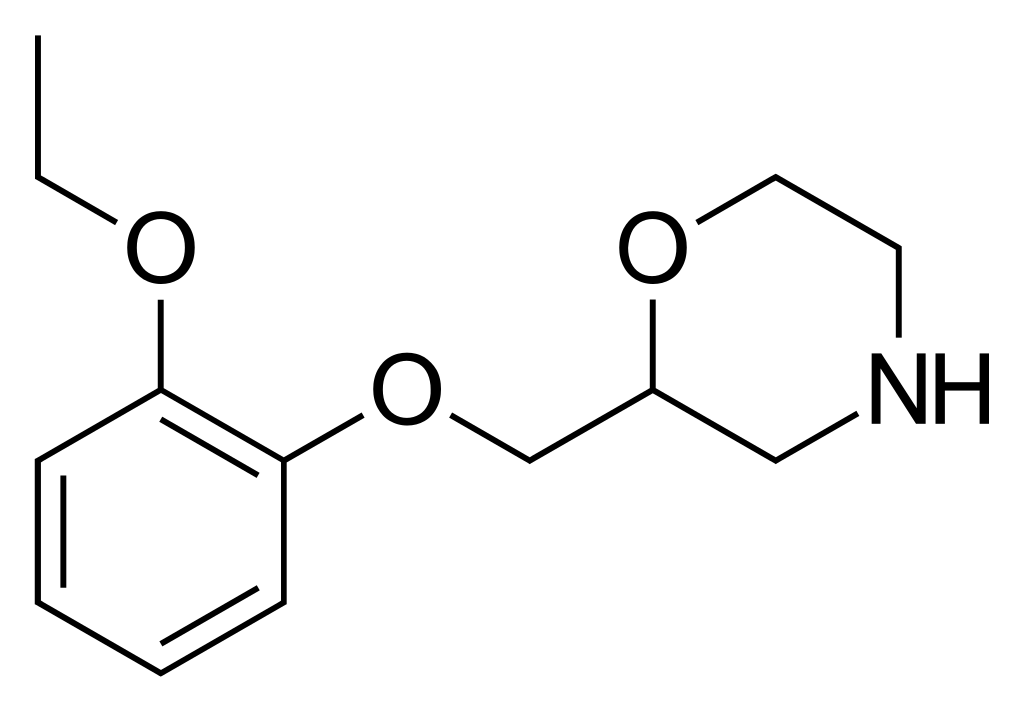
Chemistry
Viloxazine is a racemic compound consisting of two stereoisomers, with the (S)-(–)-isomer exhibiting fivefold greater pharmacological activity compared to the (R)-(+)-isomer.
History
The discovery of viloxazine can be attributed to scientists at Imperial Chemical Industries, who made a notable observation regarding certain beta blockers: at high doses, these compounds inhibited serotonin reuptake in the brain. In pursuit of enhancing their compounds’ ability to penetrate the blood-brain barrier, they made a crucial modification by replacing the ethanolamine side chain of beta blockers with a morpholine ring. This strategic alteration ultimately led to the synthesis of viloxazine, a milestone first documented in scientific literature as early as 1972.
Viloxazine made its initial debut in the market in 1974 , albeit without approval from the FDA for medical usage. In 1984, the FDA granted orphan drug status to viloxazine, designating it for the potential treatment of cataplexy and narcolepsy under the tentative brand name Catatrol. However, for reasons that remain undisclosed, it was never sanctioned or introduced for these specific purposes in the United States. Subsequently, viloxazine was globally withdrawn from the markets in 2002, a decision unrelated to concerns over its efficacy or safety.
Fast forward to 2015, Supernus Pharmaceuticals initiated the development of extended-release formulations of viloxazine for the treatment of ADHD and major depressive disorder, known as SPN-809 and SPN-812. The notable turning point came in April 2021 when viloxazine received approval in the United States for the treatment of ADHD, marking a significant milestone in its journey.
The efficacy of viloxazine was assessed through three clinical studies, encompassing two involving children aged 6 to 11 years and one involving adolescents aged 12 to 17 years, all diagnosed with ADHD. Participants in these studies were randomly assigned to receive one of two doses of viloxazine or a placebo once daily for 6 to 8 weeks. Importantly, neither the participants, their parents/caregivers, nor the study staff were aware of the treatment assignment during the trial. The results revealed that, in the last week of treatment, the severity of ADHD symptoms in participants who received viloxazine was significantly lower compared to those who received the placebo, as assessed using the Attention-Deficit Hyperactivity Disorder Rating Scale 5th Edition (ADHD-RS-5). Additionally, a fourth study provided insights into the safety profile of viloxazine in adolescents aged 12 to 17 with ADHD. The FDA approved viloxazine based on evidence from multiple clinical trials involving 1289 participants diagnosed with attention deficit hyperactivity disorder (ADHD), conducted at 59 sites across the United States.
Research
Viloxazine has been the subject of two randomized controlled trials aimed at addressing nocturnal enuresis (bedwetting) in children, both times in comparison to imipramine. As of 1990, it had garnered attention as a less cardiotoxic alternative to imipramine, particularly demonstrating efficacy in individuals with deep sleep patterns.
In the context of narcolepsy, viloxazine has exhibited the capacity to mitigate auxiliary symptoms such as cataplexy and the occurrence of abnormal sleep-onset REM episodes. However, it has shown limited improvement in daytime drowsiness levels. In a cross-over trial encompassing 56 participants, viloxazine notably reduced excessive daytime sleepiness (EDS) and occurrences of cataplexy.
Viloxazine has also been explored for its potential in treating alcoholism, yielding some positive outcomes.
However, when assessed in a double-blind, randomized controlled trial against amisulpride for the treatment of dysthymia, viloxazine demonstrated comparatively modest efficacy.
FAQ
1. What is Viloxazine? Viloxazine is a medication originally developed as an antidepressant. It is currently approved for the treatment of attention deficit hyperactivity disorder (ADHD) in the United States.
2. How does Viloxazine work? Viloxazine primarily acts as a selective norepinephrine reuptake inhibitor (NRI), affecting neurotransmitters in the brain. This mechanism is believed to contribute to its therapeutic effects in treating conditions like ADHD.
3. Was Viloxazine used for other medical conditions in the past? Yes, Viloxazine has been explored for various medical conditions. It was previously considered for the treatment of depression and was even designated as an orphan drug for cataplexy and narcolepsy. Additionally, it has been studied for its potential in addressing alcoholism.
4. Are there any side effects associated with Viloxazine? Yes, like many medications, Viloxazine can have side effects. These may include nausea, vomiting, insomnia, reduced appetite, and increased heart rate, among others. In rare cases, it may lead to thoughts of suicide or activate mania in individuals with bipolar disorder.
5. How is Viloxazine administered? Viloxazine is taken orally in the form of extended-release capsules. These capsules can sometimes be opened and sprinkled into food for easier administration.
6. Is Viloxazine a controlled substance? No, Viloxazine is not classified as a controlled substance and does not have known misuse liability.
7. Can Viloxazine be used in children and adolescents with ADHD? Yes, Viloxazine is approved for the treatment of ADHD in children aged 6 to 12 years, adolescents aged 13 to 17 years, and adults.
8. When was Viloxazine first introduced to the market? Viloxazine was first marketed in 1974, primarily as an antidepressant. However, it was later withdrawn from global markets in 2002 for commercial reasons.
9. Is Viloxazine currently available for medical use? Yes, Viloxazine was reintroduced for medical use in the United States in April 2021, specifically for the treatment of ADHD.
10. How was Viloxazine evaluated for its efficacy in treating ADHD? The efficacy of Viloxazine in treating ADHD was assessed through several clinical trials involving both children and adolescents. These trials demonstrated its effectiveness in reducing the severity of ADHD symptoms.
Please note that this information is for general reference and should not replace professional medical advice. Consult with a healthcare provider for personalized information and guidance regarding Viloxazine and its use.
References
- “Qelbree- viloxazine hydrochloride capsule, extended release”. DailyMed. Archived from the original on 28 October 2022. Retrieved 3 May 2022.
- Pinder RM, Brogden RN, Speight TM, Avery GS (June 1977). “Viloxazine: a review of its pharmacological properties and therapeutic efficacy in depressive illness”. Drugs. 13 (6): 401–421. doi:10.2165/00003495-197713060-00001. PMID 324751. S2CID 44804763.
- Case DE, Reeves PR (February 1975). “The disposition and metabolism of I.C.I. 58,834 (viloxazine) in humans”. Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 5 (2): 113–129. doi:10.3109/00498257509056097. PMID 1154799.
- “SID 180462– PubChem Substance Summary”. Archived from the original on 14 June 2013. Retrieved 5 November 2005.
- Swiss Pharmaceutical Society (2000). Swiss Pharmaceutical Society (ed.). Index Nominum 2000: International Drug Directory. Taylor & Francis. pp. 1093–. ISBN 978-3-88763-075-1. Archived from the original on 2023-01-14. Retrieved 2022-05-03.
- Cutler AJ, Mattingly GW, Jain R, O’Neal W (April 2022). “Current and future nonstimulants in the treatment of pediatric ADHD: monoamine reuptake inhibitors, receptor modulators, and multimodal agents”. CNS Spectrums. 27 (2): 199–207. doi:10.1017/S1092852920001984. PMID 33121553.
- Findling RL, Candler SA, Nasser AF, Schwabe S, Yu C, Garcia-Olivares J, et al. (June 2021). “Viloxazine in the Management of CNS Disorders: A Historical Overview and Current Status”. CNS Drugs. 35 (6): 643–653. doi:10.1007/s40263-021-00825-w. PMC 8219567. PMID 34003459.
- Mallion KB, Todd AH, Turner RW, Bainbridge JG, Greenwood DT, Madinaveitia J, et al. (July 1972). “2-(2-ethoxyphenoxymethyl)tetrahydro-1,4-oxazine hydrochloride, a potential psychotropic agent”. Nature. 238 (5360): 157–158. PMID 4558457. S2CID 4268001.
- Olivier B, Soudijn W, van Wijngaarden I (2000). “Serotonin, dopamine and norepinephrine transporters in the central nervous system and their inhibitors”. Progress in Drug Research. pp. 59–119. doi:10.1007/978-3-0348-8391-7_3. ISBN 978-3-0348-9546-0. PMID 10857386.
- Dahmen, MM, Lincoln, J, and Preskorn, S. NARI Antidepressants, pp 816-822 in Encyclopedia of Psychopharmacology, Ed. Ian P. Stolerman. Springer-Verlag Berlin Heidelberg, 2010. ISBN 9783540687061.
- Williams DA. Antidepressants. Chapter 18 in Foye’s Principles of Medicinal Chemistry, Eds. Lemke TL and Williams DA. Lippincott Williams & Wilkins, 2012. ISBN 9781609133450.
- Vignatelli L, D’Alessandro R, Candelise L (January 2008). “Antidepressant drugs for narcolepsy”. The Cochrane Database of Systematic Reviews. 2008 (1): CD003724. doi:10.1002/14651858.CD003724.pub3. PMC 9030766. PMID 18254030.
- “Qelbree: FDA-Approved Drugs”. U.S. Food and Drug Administration (FDA). Archived from the original on 2 April 2021. Retrieved 2 April 2021.
- “Supernus Announces FDA Approval of Qelbree (SPN-812) for the Treatment of ADHD”. Supernus Pharmaceuticals (Press release). 2 April 2021. Archived from the original on 6 April 2021. Retrieved 3 April 2021.
- Edwards JG, Glen-Bott M (September 1984). “Does viloxazine have epileptogenic properties?”. Journal of Neurology, Neurosurgery, and Psychiatry. 47 (9): 960–964. doi:10.1136/jnnp.47.9.960. PMC 1027998. PMID 6434699.
- Chebili S, Abaoub A, Mezouane B, Le Goff JF (1998). “[Antidepressants and sexual stimulation: the correlation]” [Antidepressants and sexual stimulation: the correlation]. L’Encephale (in French). 24 (3): 180–184. PMID 9696909.
- Pisani F, Fazio A, Artesi C, Russo M, Trio R, Oteri G, et al. (February 1992). “Elevation of plasma phenytoin by viloxazine in epileptic patients: a clinically significant drug interaction”. Journal of Neurology, Neurosurgery, and Psychiatry. 55 (2): 126–127. doi:10.1136/jnnp.55.2.126. PMC 488975. PMID 1538217.
- Perault MC, Griesemann E, Bouquet S, Lavoisy J, Vandel B (September 1989). “A study of the interaction of viloxazine with theophylline”. Therapeutic Drug Monitoring. 11 (5): 520–522. doi:10.1097/00007691-198909000-00005. PMID 2815226.
- Laaban JP, Dupeyron JP, Lafay M, Sofeir M, Rochemaure J, Fabiani P (1986). “Theophylline intoxication following viloxazine induced decrease in clearance”. European Journal of Clinical Pharmacology. 30 (3): 351–353. doi:10.1007/BF00541543. PMID 3732375. S2CID 10114046.
- Tatsumi M, Groshan K, Blakely RD, Richelson E (December 1997). “Pharmacological profile of antidepressants and related compounds at human monoamine transporters”. European Journal of Pharmacology. 340 (2–3): 249–258. doi:10.1016/s0014-2999(97)01393-9. PMID 9537821.
- Yu C, Garcia-Olivares J, Candler S, Schwabe S, Maletic V (2020). “New Insights into the Mechanism of Action of Viloxazine: Serotonin and Norepinephrine Modulating Properties”. Journal of Experimental Pharmacology. 12: 285–300. doi:10.2147/JEP.S256586. PMC 7473988. PMID 32943948.
- Richelson E, Nelson A (July 1984). “Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro”. The Journal of Pharmacology and Experimental Therapeutics. 230 (1): 94–102. PMID 6086881.
- Wander TJ, Nelson A, Okazaki H, Richelson E (December 1986). “Antagonism by antidepressants of serotonin S1 and S2 receptors of normal human brain in vitro”. European Journal of Pharmacology. 132 (2–3): 115–121. doi:10.1016/0014-2999(86)90596-0. PMID 3816971.
- Danchev ND, Rozhanets VV, Zhmurenko LA, Glozman OM, Zagorevskiĭ VA (May 1984). “[Behavioral and radioreceptor analysis of viloxazine stereoisomers]” [Behavioral and radioreceptor analysis of viloxazine stereoisomers]. Biulleten’ Eksperimental’noi Biologii I Meditsiny (in Russian). 97 (5): 576–578. PMID 6326891.
- Wermuth, CG. Analogs as a Means of Discovering New Drugs. Chapter 1 in Analogue-based Drug Discovery. Eds. IUPAC, Fischer, J., and Ganellin CR. John Wiley & Sons, 2006. ISBN 9783527607495.
- FDA. Orphan Drug Designations and Approvals: Viloxazine Archived 2022-06-25 at the Wayback Machine Page accessed August 1, 2-15.
- Bloomberg Supernus profile Archived 2018-03-11 at the Wayback Machine Page accessed August 1, 2015.
- Supernus. Psychiatry portfolio Archived 2016-04-17 at the Wayback Machine Page accessed August 1, 2015.
- “Drug Trials Snapshots: Qelbree”. U.S. Food and Drug Administration. 13 March 2023. Archived from the original on 14 March 2023. Retrieved 13 March 2023. Public Domain This article incorporates text from this source, which is in the public domain.
- Attenburrow AA, Stanley TV, Holland RP (January 1984). “Nocturnal enuresis: a study”. The Practitioner. 228 (1387): 99–102. PMID 6364124.
- Yurdakök M, Kinik E, Güvenç H, Bedük Y (1987). “Viloxazine versus imipramine in the treatment of enuresis”. The Turkish Journal of Pediatrics. 29 (4): 227–230. PMID 3332732.
- Libert MH (1990). “[The use of viloxazine in the treatment of primary enuresis]” [The use of viloxazine in the treatment of primary enuresis]. Acta Urologica Belgica (in French). 58 (1): 117–122. PMID 2371930.
- Guilleminault C, Mancuso J, Salva MA, Hayes B, Mitler M, Poirier G, Montplaisir J (1986). “Viloxazine hydrochloride in narcolepsy: a preliminary report”. Sleep. 9 (1 Pt 2): 275–279. doi:10.1093/sleep/9.1.275. PMID 3704453.
- Mitler MM, Hajdukovic R, Erman M, Koziol JA (January 1990). “Narcolepsy”. Journal of Clinical Neurophysiology. 7 (1): 93–118. doi:10.1097/00004691-199001000-00008. PMC 2254143. PMID 1968069.
- Altamura AC, Mauri MC, Girardi T, Panetta B (1990). “Alcoholism and depression: a placebo controlled study with viloxazine”. International Journal of Clinical Pharmacology Research. 10 (5): 293–298. PMID 2079386.
- Mattingly GW, Anderson RH (December 2016). “Optimizing outcomes in ADHD treatment: from clinical targets to novel delivery systems”. CNS Spectrums. 21 (S1): 45–59. doi:10.1017/S1092852916000808. PMID 28044946.